The Biogeochemical Cycles
The Sun and the natural processes within Earth provide materials and energy to the ecosystem. Aside from the occasional meteorite, there are no extraterrestrial sources of chemical elements. As such, life on Earth depends on the recycling of chemicals. Facilitating these are the different biogeochemical cycles which include both biotic and abiotic (geologic and atmospheric) components.
The cycles often have abiotic reservoirs, where the chemicals accumulate or are stored outside of living organisms. The atmosphere, for example, is a reservoir for carbon while phosphorus is available only from the soil. Both atmosphere and soil are reservoirs of nitrogen.
In general, the biogeochemical cycles require producers to incorporate the chemicals from the reservoir into organic compounds that are then transferred to the consumers. Both producers and consumers release some of the chemicals back to the environment as waste products while decomposers play a central role in breaking down complex organic molecules into inorganic compounds that replenishes the abiotic reservoirs.
Geologic processes such as erosion and weathering also contribute to the reservoirs. Biogeochemical cycles can be local or global. For example, gaseous chemicals are recycled on a global scale.
We will elaborate more on the cyclic movement of carbon, phosphorus, and nitrogen.
1. Carbon Cycle
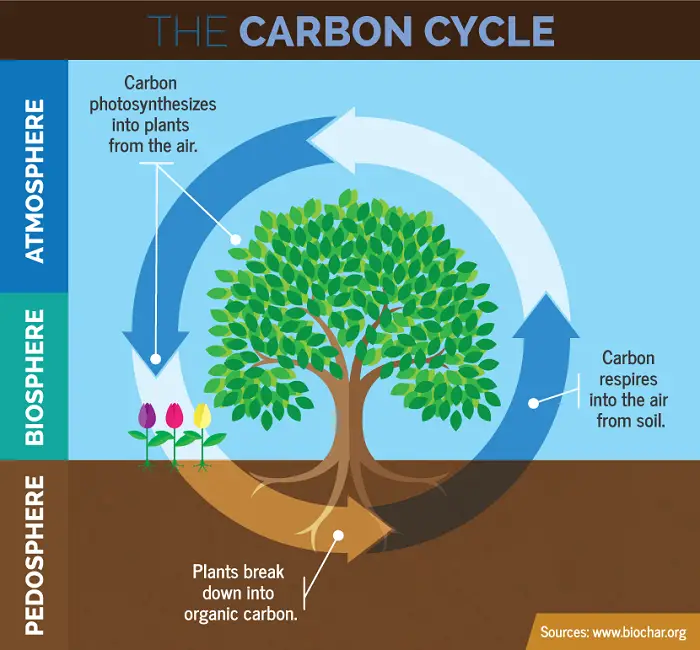
Carbon is an important component of organic molecules. The processes of photosynthesis and cellular respiration are mainly responsible for recycling carbon between the biotic and abiotic components.
Photosynthesis removes CO2 from the atmosphere and transforms it into organic molecules. The molecules are passed on the food chain through consumers.
Cellular respiration by producers and consumers returns CO2 to the atmosphere. Decomposers also break down carbon compounds in detritus that eventually return to the atmosphere as carbon dioxide. This is why the burning of fossil fuels must be regulated as the carbon they store is released back as carbon dioxide in the atmosphere and can lead to an increase in global temperatures.
2. Phosphorus Cycle
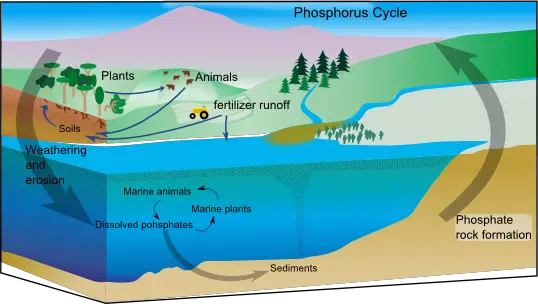
Phosphorus is found in cells as they are an ingredient of nucleic acids, phospholipids, and ATP. Rocks are the only source of phosphorus for terrestrial ecosystems and so, they are mined for agricultural fertilizer.
The weathering of rock adds inorganic phosphate (PO43-) to the soil. Plants assimilate the dissolved phosphate ions and build them into organic compounds. Consumers obtain phosphorus by eating plants. Phosphates are returned to the soil by decomposers on animal wastes and the remains of dead animals and plants. Some phosphate drains into the sea and may settle to become part of new rocks. This phosphorus will not be able to return back to living organisms until geologic processes uplift the rocks and expose them to weathering processes.
Because phosphates move to aquatic ecosystems more rapidly than they return to the terrestrial environment, this also contributes to the low availability of phosphate and so it can become a limiting factor to some organisms.
3. Nitrogen Cycle
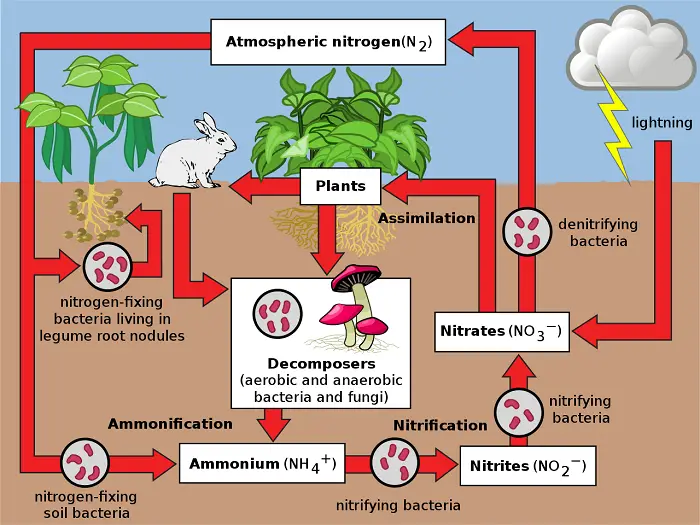
Nitrogen is an ingredient of proteins and nucleic acids that is why it is important to all organisms. In particular, it often limits plant nutrients.
Nitrogen has two abiotic reservoirs: the atmosphere and the soil. Almost 80% of the atmosphere is nitrogen gas (N2) but this cannot be absorbed by plants. Nitrogen fixation is required to convert it into compounds that can be used by plants. Since this process is performed by bacteria, they are important to the cycle.
Some bacteria live symbiotically in the roots of certain plants, most notably legumes, while others are free-living in soil or water. These bacteria convert N2 to ammonia (NH3) which then picks up another H+ to become ammonium (NH4+).
After fixation, some of the NH4+ is taken up by plants while some are converted into nitrate (NO3–) by nitrifying bacteria. Nitrates are more easily assimilated by plants to synthesize molecules such as amino acids. When a herbivore eats a plant, it digests the proteins into amino acids to build the proteins it needs. The succeeding orders of consumers get nitrogen from their prey. Some nitrogen is incorporated into tissues while metabolism produces nitrogen-containing waste that is then excreted by the consumer.
When organisms die, they decompose and release NH4+ from organic compounds back into the soil, replenishing the soil reservoir (recall there are also nitrifying bacteria). Under low-oxygen conditions, however, soil bacteria known as denitrifiers remove the oxygen atoms from NO3- and release the N2 back into the atmosphere and depleting the soil reservoir of usable nitrogen.
The succeeding topics will wrap up and connect everything we have learned and will conclude the entirety of the reviewer. Our last subtopic revolves around the issues faced by different species and the matter of conservation and restoration.
Next topic: Conservation Biology and Restoration Ecology
Previous topic: Ecosystem Structure and Dynamics
Return to the main article: The Principles of Ecology
Download Article in PDF Format.
Test Yourself!
1. Practice Questions [PDF Download]
2. Answer Key [PDF Download]
Copyright Notice
All materials contained on this site are protected by the Republic of the Philippines copyright law and may not be reproduced, distributed, transmitted, displayed, published, or broadcast without the prior written permission of filipiknow.net or in the case of third party materials, the owner of that content. You may not alter or remove any trademark, copyright, or other notice from copies of the content. Be warned that we have already reported and helped terminate several websites and YouTube channels for blatantly stealing our content. If you wish to use filipiknow.net content for commercial purposes, such as for content syndication, etc., please contact us at legal(at)filipiknow(dot)net