Matter

Chemistry is the study of matter and the changes it undergoes. By definition, matter is anything that occupies space and has mass. That being said, anything that you can think about is probably made up of matter and hence, can be studied in chemistry. In this section, we will discuss the properties, states, and types of matter.

Click below to go to the main reviewers:
Table of Contents
- Properties of Matter
- Types of Matter
- States of Matter
- References
- Download Article in PDF Format
- Test Yourself!
Properties of Matter
All matter has physical and chemical properties. If that property can be measured without changing the chemical composition of that matter, it is a physical property. The physical properties of matter can be further subdivided into extensive and intensive properties.
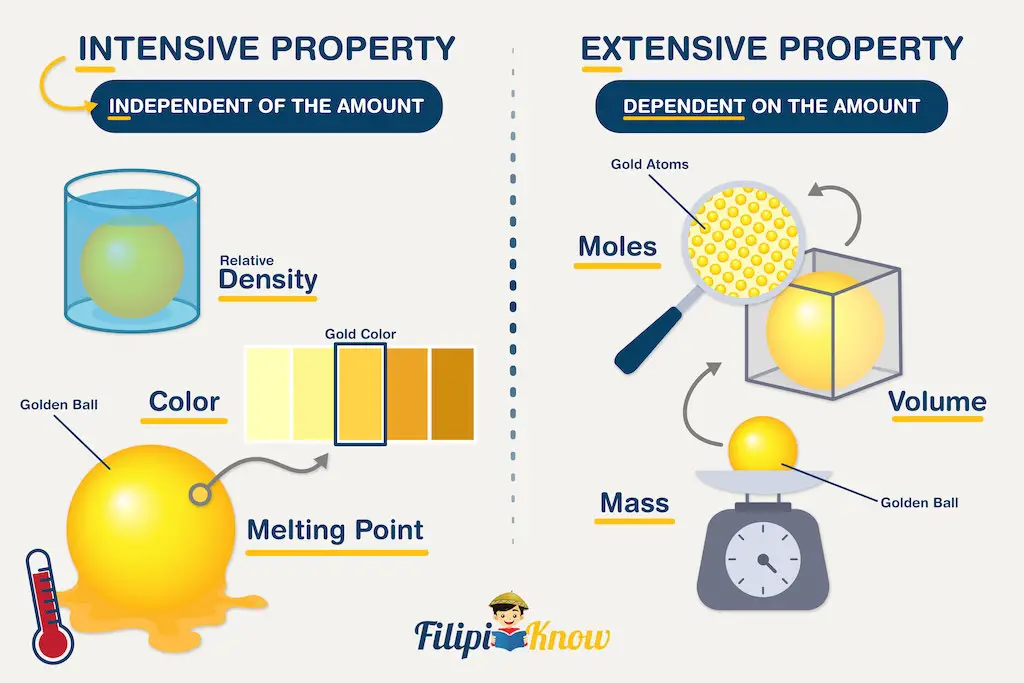
Extensive properties are those that depend on the amount of matter being measured. The classic examples of extensive physical properties are the mass, volume, and number of moles. Decreasing the amount of matter being weighed will decrease the mass of that matter.
On the other hand, intensive properties are independent of the amount of matter being considered. Examples of intensive properties are color, melting point, boiling point, and density. The density of water, regardless of whether you use 1 mL or 1 L of it, will be the same at a certain temperature.
Meanwhile, chemical properties describe the characteristic ability of a substance to react to form new substances. Classic examples of chemical properties include flammability and susceptibility to corrosion.
Types of Matter
Matter can be classified into two general categories: pure substance or mixture. The diagram below summarizes the differences between the two types of matter.
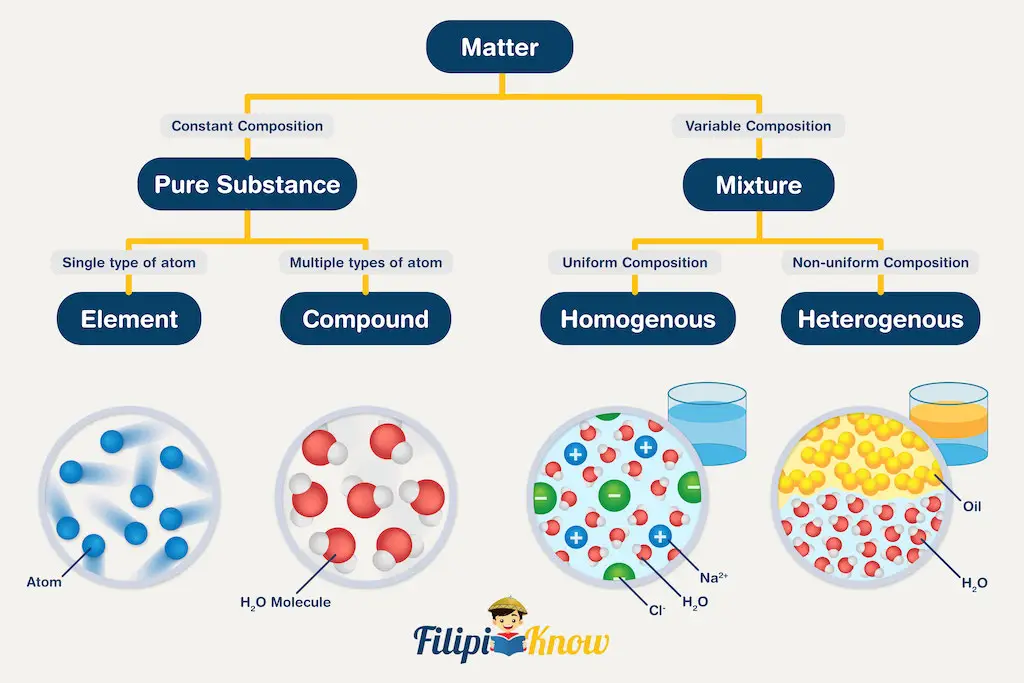
1. Pure substance
A substance is a form of matter with a definite composition and distinct properties. A pure substance can be further classified into two categories: elements and compounds.
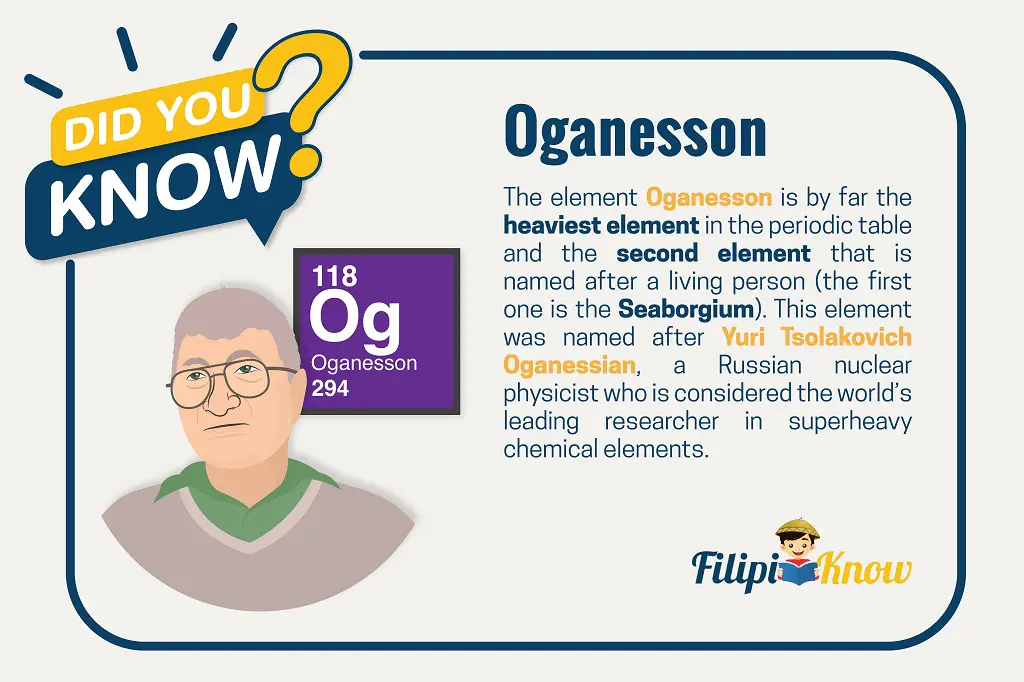
A substance that cannot be broken down into simpler substances using any chemical means is known as an element. With the successful synthesis of Oganesson (Og), there are 118 known elements to date. The introduction of Oganesson also completes the modern periodic table. Scientists are currently on their way to extending the periodic table of the elements.
When two or more types of elements are used in fixed proportion with one another to form a single substance, a compound is formed.
2. Mixture
The other general classification of the matter is mixture. A mixture is a combination of two or more substances in which the substances retain their distinct identities.
Mixtures can be further subdivided into heterogeneous and homogeneous mixtures. In a homogeneous mixture, the composition of the mixture is the same throughout. In other words, if you cannot recognize the individual components of a mixture, then it is homogeneous. For example, dissolving a small amount of table salt in one glass of water results in a homogeneous mixture we usually call a solution.
The solution is composed of two components: the solute(s) and the solvent. The component that exists in the greatest amount is the solvent, while solute is/are the component/s that exists in minor amount/s. When studying the chemistry of solutions, you will often encounter the word “aqueous.” In most cases, an aqueous solution means that water exists in the greatest proportion in the solution and hence, acts as a solvent.
Meanwhile, If you can differentiate the components of a mixture, then it is a heterogeneous mixture. Heterogeneous mixtures can be further subdivided into suspension and colloids.
Adding a scoopful of sand to a glass of water results in a mixture where you can distinguish sand from water (since water cannot dissolve sand). The sand-water mixture is a suspension.
Colloids, on the other hand, are quite tricky because, at first glance, it appears as if they are solutions. Let’s take for example a glass of fresh milk. At first glance, milk appears as a solution since we know that milk is made up of multiple components, but we can only see a single liquid phase. However, under the microscope, milk appears as globules suspended in a certain liquid matrix. Milk behaves like this because it is made of fats and water, and we know these two do not mix completely.
If that is the case, then how do suspension and colloid differ from one another? In terms of size, particles in the suspension are so large that eventually, the particles settle at the bottom. In contrast, colloids are composed of particles that are small enough (usually from 1 to 1000 nanometers) for them to remain dispersed in their matrix.
When studying the chemistry of colloids, you will often hear the terms dispersed and continuous phase. The component/s of the mixture that is/are being dispersed is the dispersed phase, while the component where it is being dispersed is the continuous phase or the dispersion medium.
Aside from microscopy, there is another simpler way to differentiate colloids from solutions. This is done by just using a flashlight! When a beam of light is passed through a colloid, the dispersed phase scatters it. This phenomenon is known as the Tyndall effect. Solutions do not exhibit this scattering effect; instead, you will see a clean transmission of light from one end of the glass to the other.

Lastly, there are different colloids depending on the nature of the dispersed and continuous phases. You can also encounter the term “hydrocolloid,” a colloidal system wherein water acts as the dispersion medium.
| Colloid Type | Dispersed Phase | Continuous Phase | Example |
| Solid sol | Solid | Solid | steel, opal |
| Solid emulsion or Gel | Liquid | Solid | butter, cheese |
| Solid foam | Gas | Solid | plastic foam, lava |
| Sol | Solid | Liquid | milk of magnesia, paint |
| Emulsion | Liquid | Liquid | mayonnaise, milk |
| Foam | Gas | Liquid | whipped cream, soap suds |
| Solid Aerosol | Solid | Gas | smoke |
| Liquid Aerosol | Liquid | Gas | fog, mist |
States of Matter
Recent advances in science allow us to classify states of matter more meticulously. To demonstrate how far we’ve come, there are 22 known states of the matter today, and more are expected to be discovered in the years to come. However, in this learning material, we will focus on the four most basic states of matter (yes, four, not three!) and what separates them. The figure below gives you a headstart!
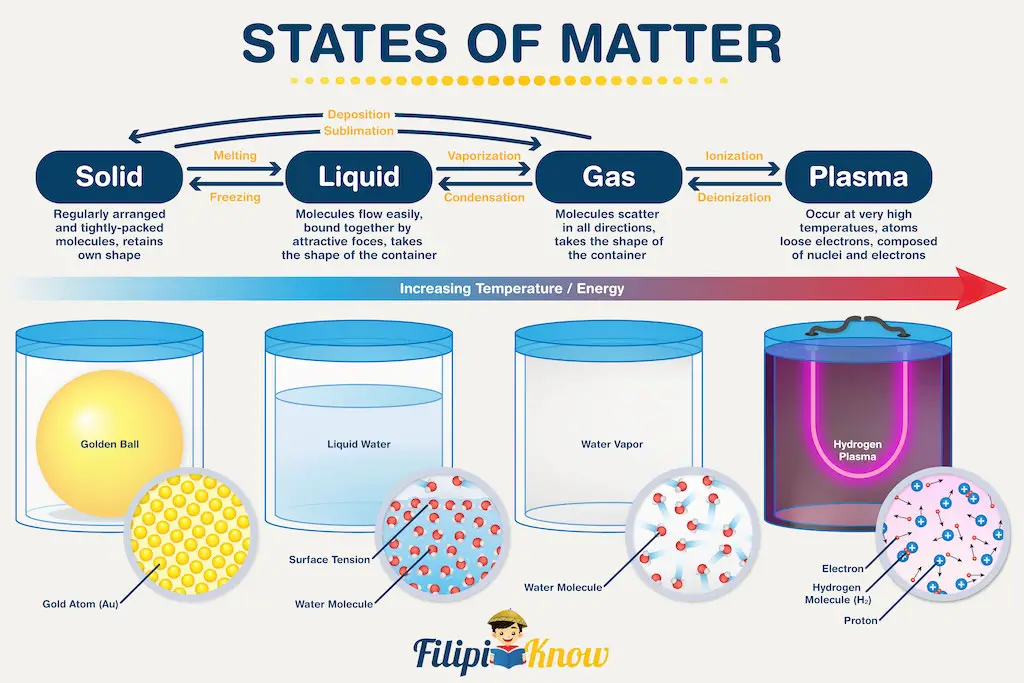
Let’s start with solids. During our elementary days, we were taught that solids have their shape and do not occupy the shape of their container. That’s because solids are made up of molecules (or atoms) arranged in a regular, repeating manner. Furthermore, the molecules or atoms are fixed in their position, which confers shapes to solids.
Liquid and gases, on the other hand, have molecules (or atoms) that can move freely, although liquid molecules are more compact and hence, have more restricted movement. One major consequence of this property is the lack of shapes of liquids and gases, therefore they are dubbed as the states of matter that copy the shape of their container.
Now, you are probably least familiar with the 4th state of matter which is plasma. In my elementary and high school days, plasma was not being taught as a state of matter (Can you guess my age now?). To put it simply, plasma is just like gas; however, the difference is that gases are made up of neutral molecules or atoms, while plasma is made up of charged molecules or atoms. In other words, you can say that plasma is a charged gas!
For plasma to occur though, extremely harsh conditions are required. As a result, we can only observe plasma on the surface of very hot objects, such as the stars.
References
Chang, R. (2010). Chemistry (10th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Colloids. (2020). Retrieved 9 March 2022, from https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Solutions_and_Mixtures/Colloid
Emspak, J. (2016). States of Matter: Plasma. Retrieved 9 March 2022, from https://www.livescience.com/54652-plasma.html
Silberberg, M. (2006). Chemistry: The molecular nature of matter and change (4th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Zumdahl, S., Zumdahl, S., & DeCoste, D. (2010). Chemistry (8th ed.). Belmont, California: Brooks Cole.
Next topic: Atoms
Return to the main article: The Ultimate Chemistry Reviewer
Download Article in PDF Format
Test Yourself!
1. Practice Questions [PDF Download]
2. Answer Key [PDF Download]
Written by John Bryan Rolloque
in College Entrance Exam, LET, NMAT, Reviewers, UPCAT
John Bryan Rolloque
John Bryan Rolloque graduated cum laude at the University of the Philippines Los Baños in 2018 under the B.S. Agricultural Chemistry program. He taught courses in general chemistry, analytical chemistry, and organic chemistry at UPLB’s Institute of Chemistry, and has been serving as the Region IV coordinator for the Regional and National Chemistry Olympiad. Landing 8th place in the 2019 licensure exam for agriculturists, he is now taking up his master’s degree in plant physiology, also in UPLB.
Copyright Notice
All materials contained on this site are protected by the Republic of the Philippines copyright law and may not be reproduced, distributed, transmitted, displayed, published, or broadcast without the prior written permission of filipiknow.net or in the case of third party materials, the owner of that content. You may not alter or remove any trademark, copyright, or other notice from copies of the content. Be warned that we have already reported and helped terminate several websites and YouTube channels for blatantly stealing our content. If you wish to use filipiknow.net content for commercial purposes, such as for content syndication, etc., please contact us at legal(at)filipiknow(dot)net