Biomolecules
Living beings are composed of matter manifesting in the form of atoms, molecules, and compounds. One must wonder how these things which are invisible to the naked eye hold great sway over the complexity that is life. This reviewer will help you refresh your memory of the biomolecules–carbohydrates, lipids, proteins, and nucleic acids–that are considered building blocks of life.
Click below to go to the main reviewers:
Table of Contents
- An Introduction to Biomolecules
- 1. Carbohydrates
- 2. Lipids
- 3. Proteins
- 4. Nucleic Acids
- Summary
- References
- Download Article in PDF Format
- Test Yourself!
An Introduction to Biomolecules
Different cultures and religions tell stories of how the world came to be.
In the field of science, this translates to an event theorized to have occurred billions of years ago and birthed everything we perceive today. The matter came to be with that event and for Earth, our planet is just in the right spot and conditions to help flourish life as we know it.
Life on earth is based on the element carbon. The most important characteristic of carbon as the basis for the chemistry of life is its capability to form up to four valence bonds with other atoms simultaneously, and the energy needed to create or break a bond with a carbon atom is at a level appropriate enough for building large and complex molecules which are stable and reactive.
Carbon atoms also bond readily with other carbon atoms through the process known as catenation, allowing the creation of long macromolecules and polymers. Per Stephen Hawking: “What we normally think of as life is based on chains of carbon atoms.”
Carbon is capable of forming vast numbers of compounds than any other element, ten million described to date and maybe even more.
To recall, the cell is the functional unit of life. The most abundant element in cells is hydrogen (H), followed by carbon (C), oxygen (O), nitrogen (N), phosphorus (P), and sulfur (S). These elements are called macronutrients. Some elements are required by some cells in very small amounts and are called micronutrients or trace elements. All of these elements are essential to the function of many biochemical reactions, and therefore, are essential to life.
A big chunk of carbon-containing compounds has led to a distinction between organic and inorganic molecules or compounds (the latter do not contain carbon). Organic compounds in organisms are generally larger and more complex than inorganic compounds. All these molecules are called biomolecules because they are part of living matter and contain carbon, which is the building block of life.
In addition to carbon, biomolecules also contain functional groups—groups of atoms within molecules categorized by their specific chemical composition and the chemical reactions they perform, regardless of the molecule in which the group is found. Carbon chains form the skeletons of most organic molecules. Functional groups combine with the chain to form biomolecules.
Because these biomolecules are typically large, they are also called macromolecules. Many macromolecules are formed by linking together identical, or very similar, smaller organic molecules. The smaller molecules act as building blocks and are called monomers, and the macromolecules that result from their linkage are called polymers.
Cells link monomers to form polymers by means of a dehydration reaction, a reaction that removes water as two molecules are bonded together. Not only must cells make macromolecules but they must also have to break them down. Imagine how our food is able to energize us, our body is able to digest the larger food compounds into bits and chunks before they can be distributed throughout the body, providing energy.
This digestion is called hydrolysis and is essentially a reverse to dehydration reaction, in that water helps break the bond between molecules hence why it feels easier to eat while drinking fluids. Both reactions require the help of enzymes to make and break the bonds. Enzymes are specialized macromolecules that speed up reactions in cells. In cases of people with lactose intolerance, they lack the enzymes needed to break down the sugar lactose.
Cells and cell structures include four main groups of carbon-containing macromolecules: carbohydrates, lipids, proteins, and nucleic acids.
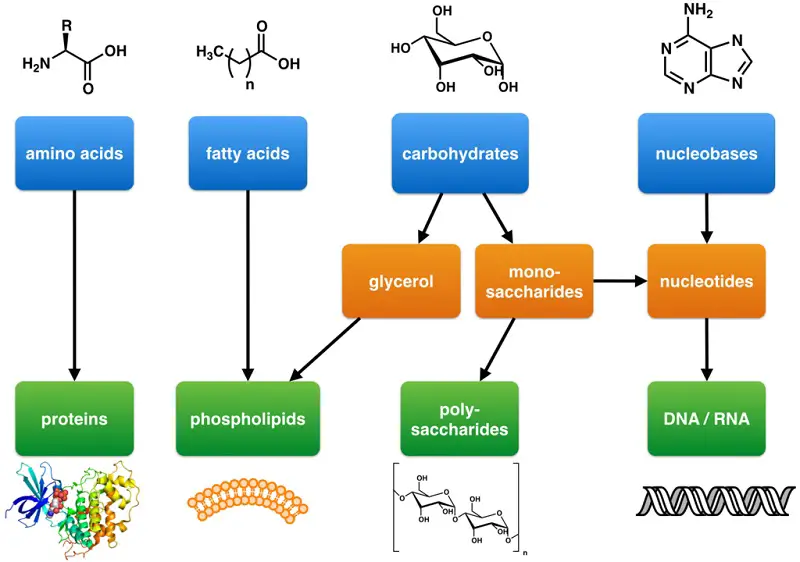
1. Carbohydrates
Let’s start our survey of biological molecules with carbohydrates.
This class of molecules ranges from small sugar molecules to large polysaccharides. Simple sugars or monosaccharides are the monomers of carbohydrates. They generally have a molecular formula that is some multiple of CH2O.
For example, glucose, the sugar most important to live, is C6H12O6 (6 times the CH2O). Because molecules can be arranged, it is possible that biomolecules contain the same number of elements but have different structures; these are called isomers.
For example, fructose also has the chemical formula C6H12O6, but it differs structurally from glucose. Slight changes in structure give isomers different properties, most notably how they would react with other molecules. This difference for instance is why fructose is much sweeter than glucose.

The carbon skeleton of glucose and fructose is six carbon atoms long, but other monosaccharides may have three to seven carbons. Five-carbon sugars, pentoses, and six-carbon sugars, hexoses, are among the most common.
Sugars are often drawn as if their carbon skeletons are linear, but in aqueous solutions, most five- and six-carbon sugars form rings.
Earlier, we mentioned an enzyme that breaks down the sugar lactose. That enzyme is called lactase. Note that most names for sugars end in -ose and for enzymes, end in -ase.
Monosaccharides, particularly glucose, are the main fuel for cellular work. Energy is released when bonds are broken and glucose, due to its simple structure, provides an immediate energy source to cells. Cells also make use of their carbon skeleton as material for making other kinds of organic molecules.
When two monosaccharide monomers are linked by means of a dehydration reaction, a disaccharide is formed. Sucrose is the most common disaccharide, and it is made by joining glucose with fructose. Transported in the plant sap, sucrose provides energy and raw materials to all parts of the plant. We can extract sucrose from the stems of sugarcane or roots of sugar beets to use as table sugar.
Polysaccharides are macromolecules, polymers of hundreds to thousands of monosaccharides linked by dehydration reactions. They may function as storage molecules or as structural compounds. Starch is a storage polysaccharide in plants consisting of chains of glucose. These glucose monomers may coil into a helical shape and be unbranched or branched. Starch granules serve as carbohydrate reserves from which plant cells can withdraw glucose.
Humans and most other animals have enzymes that can hydrolyze starch to glucose. Potatoes and grains are major sources of starch in the human diet. Animals store glucose as a polysaccharide called glycogen. In the body, glycogen is stored as granules in the liver or our muscle cells. The glycogen is hydrolyzed to release glucose when it is needed.
Another polysaccharide is a major component of the tough walls that enclose plant cells, cellulose. It is the most abundant organic compound on Earth, and how its monomers are arranged allows it to form cable-like microfibrils. These microfibrils, combined with other polymers, produce strong support for trees and the structures we build with lumber.
As we do not have enzymes that can break the glucose bonds in cellulose, it is not a nutrient for us, but it still contributes to our digestive health as “fiber.” Fresh fruits, vegetables, and whole grains are rich in fiber.
Insects and crustaceans build their exoskeletons, the hard enclosing case of the animal, with the polysaccharide chitin. Chitin also makes up the cell walls of fungi.

Almost all carbohydrates are hydrophilic or water-loving, due to the way many hydroxyl groups are attached to their sugar monomers. This is why cotton bath towels, made mostly of cellulose, are water absorbent. But as you’ll see next, not all biomolecules “love water.”
2. Lipids
Lipids are a diverse group of molecules with one trait: they don’t mix well with water.
They are thus hydrophobic, and you can see their chemical behavior when you mix oil with water or how some fat floats in a broth.
Lipids differ from other biomolecules in that they are neither huge macromolecules nor are they polymers built from similar monomers. Three important types of lipids we will delve deeper into are fats, phospholipids, and steroids.
Fat is a large lipid made from two types of smaller molecules: glycerol and fatty acids. Glycerol consists of three carbons, each having a hydroxyl group (-OH). A fatty acid consists of a carboxyl group (-COOH) and a hydrocarbon chain. The hydrocarbon chain is the reason fats are hydrophobic. In a way, you can consider the glycerol as the head, and because the hydrocarbon chain is lengthy, it can be referred to as the tail.
A fatty acid whose hydrocarbon chain has one or more double bonds is called an unsaturated fatty acid. These double bonds cause kinks (or bends) in the carbon chain. If a fatty acid lacks this double bond, all its hydrocarbon chain contains the maximum number of hydrogens attached per carbon atom (hence it is “saturated” with hydrogens) and is called a saturated fatty acid.

Most animal fats are saturated; the tails of their fatty acids lack double bonds, making them pack closely together. It also explains why they are solid at room temperature. In contrast, plant fat and those from fish contain unsaturated fatty acids – the kinks in their tails prevent them from packing tightly together. Thus, unsaturated fats are usually liquid at room temperature and are referred to as oils.
On some food labels, you may have seen “partially hydrogenated oils” which means that unsaturated fats have been converted to saturated fats by adding hydrogen. Unfortunately, this process also creates trans fats which have associated health risks.
The main function of fat is energy storage. A gram of fat, for instance, stores twice as much energy as a gram of polysaccharide. For immobile plants, starch being the main energy storage compound is not a problem. Mobile animals get around more and carry their energy reserves as fat. Of course, the downside to this energy-storing mechanism is that it takes much more effort to “burn off” the excess.
A reasonable amount of body fat is both normal and healthy. These long-term fuel reserves are stored in our body in adipose cells, which shrink or swell as we withdraw or deposit fat from them. Aside from energy storage, fatty tissues cushion vital organs and insulate our body from the cold.
Cells could not exist without phospholipids, the major components of cell membranes. They are similar in structure to fats except that only two fatty acids are attached to the glycerol instead of three. The last carbon in glycerol is attached to a negatively charged phosphate group.
The two ends of a phospholipid have different relationships with water, resulting in a double-layered sheet arrangement in a watery environment. The hydrophobic tails of the phospholipids clump together in the center of the sheet while the hydrophilic phosphate heads face the watery environment on either side of the resulting membrane.
Steroids are lipids where the carbon skeleton contains four fused rings. Cholesterol is a common component in animal cell membranes and is also a precursor for making other steroids, including sex hormones. The steroids we often hear as bad news are the anabolic steroids, synthetic variants of testosterone. Testosterone helps build muscle and bone mass during puberty and contributes to masculine traits throughout life. Because anabolic steroids resemble testosterone, it mimics its effects on the body.
When regulated or prescribed by a medical professional, these steroids help treat anemia and diseases that destroy the muscle. But in the case of substance abuse, it may lead to violent mood swings (“roid rage”), depression, liver damage or cancer, and high cholesterol levels and blood pressure. The use of these substances reduces the output of natural testosterone in the body which can cause shrunken testicles, reduced sex drive, infertility, and breast enlargement in men.
3. Proteins
Nearly every action in the body depends on proteins.
A protein is a polymer composed of blocks of amino acids. Proteins are the most structurally and functionally elaborate and varied among the biomolecules. Probably their most important role with regards to cells is those acted by enzymes; that is they catalyze or help speed up and regulate virtually all chemical reactions in cells.
Other proteins act for transport, structural support, and storage. The functions of these different types of proteins depend on each protein’s unique shape.
Most enzymes and other proteins are globular proteins. Structural proteins that make up the hair, tendons, and ligaments are long and thin and called fibrous proteins. Descriptions such as globular and fibrous refer to a protein’s general shape.
Each protein has a much more specific shape. Nearly all proteins must recognize and bind to some other molecule to function, and the shape of proteins allows them to be more specific when binding with other molecules.
The dependence of function on the protein’s shape is clear when a protein is altered. In a process called denaturation, a protein unravels and loses its specific shape and, as a result, its function.
Heat is one factor that leads to denaturation. Visualize an egg that is being fried. The clear proteins surrounding the yolk become solid, white, and opaque during the frying process.

In the cell, newly formed amino acid chains fold into a normally functioning protein. When the protein doesn’t fold correctly, the disease may develop. Examples are Alzheimer’s and Parkinson’s diseases which involve the accumulation of misfolded proteins. Prions are infectious misshapen proteins associated with serious degenerative brain diseases. Such diseases highlight the correlation between form and function.
Amino acids have an amino group and a carboxyl group bonded to a carbon atom. With carbon being able to bind to four other molecules, the other two partners of this carbon are hydrogen and a variable group symbolized by the letter R.
Cells join amino acids in a dehydration reaction that links the carboxyl end of one amino acid to the amino end of the next amino acid. This link is called a peptide bond. Additional amino acids added to the chain through the same process make a chain called a polypeptide.
There are 20 amino acids that vary depending on the R group (with each R group possibly being nonpolar, polar, or charged), but it is possible to create thousands of different kinds of protein from just these 20 amino acids.
Imagine the amino acids as “letters” of an alphabet, polypeptides are the “words” of any language which vary based on how the letters are sequenced or how lengthy a word can get. Whereas words are composed of letters arranged in a specific sequence, polypeptides have a unique sequence of amino acids.
However, a long polypeptide chain is not the same as a protein as much as a long yarn is not the same as a sweater, not until you knit that yarn into a sweater. The “stitches” that allow a polypeptide chain to form unique three-dimensional chains are because of the R groups and their contribution to influencing protein structure.
Hydrophobic amino acids may cluster together in the center of a globular protein, while the hydrophilic amino acids face outside, helping proteins dissolve in the aqueous environment of the cell. The type of bonds in the R group help determine the protein’s shape. Ultimately, the unique sequence of the various types of amino acids in a polypeptide chain determines how a protein takes shape.
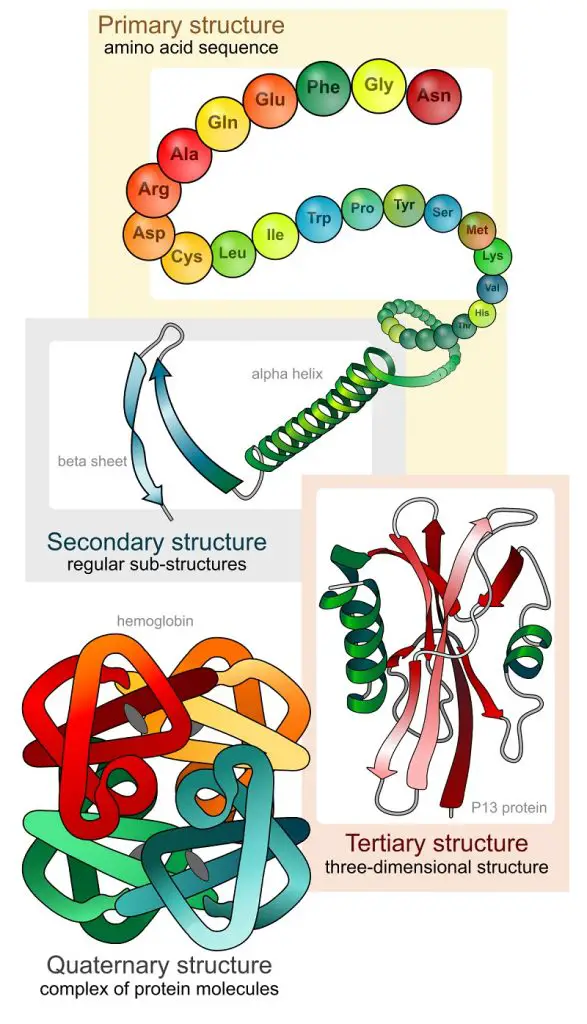
The primary structure of a protein is this precise sequence of amino acids in a polypeptide chain. When the chain coils into local patterns, a secondary structure is formed. Among these patterns are the alpha helix and the beta-pleated sheet. When a protein becomes more three-dimensional, the protein takes on a tertiary structure. When more than one polypeptide chain contributes to the shape of a protein, a quaternary structure is formed.
4. Nucleic Acids
Earlier, we mentioned that the sequence of amino acids determines the shape of a protein. But what determines this specific sequence?
The amino acid sequence is programmed by a discrete unit of inheritance, the gene. The gene consists of DNA (deoxyribonucleic acid), one of two types of polymers called nucleic acids. The DNA is located in the nucleus of cells. The other polymer is RNA (ribonucleic acid) which plays a role in assembling polypeptides according to the instructions of DNA.

The monomers that make up nucleic acids are nucleotides. Each nucleotide is composed of three parts: a five-carbon sugar in the center with one end linked to a phosphate group and the other end linked to a nitrogenous base, a molecule containing nitrogen and carbon. The sugar in DNA is deoxyribose while those in RNA are slightly different, ribose.
Each DNA nucleotide can have one of four possible nitrogenous bases: adenine (A), thymine (T), cytosine (C), and guanine (G). Thus, it can be said that all genetic information is written with a four-letter alphabet. Meanwhile, RNA nucleotides also contain A, C, and G bases but in place of thymine is the base uracil (U).
RNA is single-stranded while DNA molecules wind around each other forming a double-stranded helix. Because of their structure, nucleotide bases bond with a specific base with A always pairing with T, and C pairs with G. The two DNA strands are held together by hydrogen bonds between specific nucleotide bases.
Individually, the bonds are weak, but collectively the strands are held as a stable double helix. Because of the base pairing, the two strands of DNA are complementary. Thus, you can determine the other strand when given the sequence of the other.
The genetic material that all organisms inherit from their parents consists of DNA. DNA is located in the cell as chromosomes, each carrying several genes. Unique among biomolecules, DNA provides direction for its replication.
Each time a cell divides, it first makes two identical copies of each of its chromosomes. Why is the DNA’s structure important to this process? As mentioned before, the complementary base pairing holds the key – the double helix unzips, and new complementary strands assemble along the separated strands.
Thus, as the cell divides, its genetic instructions are passed to each daughter cell. These instructions program all of the cell’s activities and direct the synthesis of proteins. The roles of DNA and RNA in producing proteins are referred to as gene expression.
Summary
| Biomolecule | Functions |
| Carbohydrates | Energy storage, receptors, food, structural role in plants and fungi cell walls, exoskeletons of insects |
| Lipids | Energy storage, membrane structure, insulation, hormones, pigments |
| Nucleic Acids | Storage and transfer of genetic information |
| Proteins | Enzymes, structure, receptors, transport, structural role in the cytoskeleton of a cell, and the cellular matrix |
We have established the different building blocks that are essential to forming life. In the succeeding topics, we will expand into other aspects and properties of life, starting with the most basic unit of it: the cell.
References
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular biology of the cell. New York: Garland Science.
Nelson, D. L., Cox, M. M., & Lehninger, A. L. (2017). Lehninger principles of biochemistry. New York, NY: W.H. Freeman and Company.
Openstax. (2020). Microbiology. Openstax. Retrieved 8 November 2020 from https://openstax.org/books/microbiology/pages/1-introduction
Reece, J. B., & Campbell, N. A. (2011). Campbell Biology. Boston: Benjamin Cummings/Pearson.
Stephen Hawking (1 October 2008). “Life in the Universe, 50th-anniversary celebration of NASA”. NASA. Retrieved 8 November 2020.
Taylor, M. R., Simon, E. J., Dickey, J., Hogan, K. A., & Reece, J. B. (2018). Campbell Biology: Concepts & connections. NY: Pearson.
Next topic: The Cell
Previous topic: Properties of Life
Return to the main article: The Ultimate Biology Reviewer
Download Article in PDF Format
Test Yourself!
1. Practice Questions [PDF Download]
2. Answer Key [PDF Download]
Written by Earl Jeroh Bacabac
in College Entrance Exam, LET, NMAT, Reviewers, UPCAT
Earl Jeroh Bacabac
Earl’s love for the sea fueled his goal to become a marine biologist. He obtained his Bachelor’s Degree in Biology from the University of the Philippines Visayas while also being a DOST scholar. His passion for the marine environment is rivaled by his diverse interests in music, the arts, and video games.
Copyright Notice
All materials contained on this site are protected by the Republic of the Philippines copyright law and may not be reproduced, distributed, transmitted, displayed, published, or broadcast without the prior written permission of filipiknow.net or in the case of third party materials, the owner of that content. You may not alter or remove any trademark, copyright, or other notice from copies of the content. Be warned that we have already reported and helped terminate several websites and YouTube channels for blatantly stealing our content. If you wish to use filipiknow.net content for commercial purposes, such as for content syndication, etc., please contact us at legal(at)filipiknow(dot)net