Nuclear Chemistry

In earlier modules, we discussed chemical reactions involving the electrons of different atoms. But, did you know that atomic nuclei can also participate in a chemical reaction? This is the realm of nuclear chemistry.
Nuclear chemistry began when Antoine Henri Becquerel, a French engineer and physicist, discovered the first evidence of radioactivity, a phenomenon in which unstable nuclei emit particles or electromagnetic radiation spontaneously.
Marie Sklodowska Curie is also one of the scientists who pioneered research on radioactivity. Two of her most notable accomplishments are the discovery of polonium (Po) and radium (Ra). Her dedication to this field of study led to Curie succumbing to aplastic anemia, which is linked to overexposure to radioactive elements she was working on. Marie Curie’s body was so radioactive that her coffin was lined with almost one-inch thick lead.
Furthermore, Curie’s belongings, especially her laboratory notebook (shown above) and manuscript, are so radioactive that they’re being stored in lead-lined boxes at France Bibliotheque Nationale in Paris. Even so, guests who want to see these documents are required to sign a liability waiver and wear protective gear. Her belongings are expected to remain radioactive for the next 1500 years!
Learn more about nuclear chemistry in this module.
Click below to go to the main reviewers:
Table of Contents
- Notations in Nuclear Chemistry
- Balancing Nuclear Reactions
- Calculation of Half-Life
- References
- Download Article in PDF Format
- Test Yourself!
Notations in Nuclear Chemistry
So far, we have only concerned ourselves with electrons in an atom since they’re greatly involved in chemical reactions. In nuclear chemistry, we will focus more on the protons and neutrons in an atom.
For most purposes, the nucleus of an atom can be regarded as a collection of nucleons (neutrons or protons). A distinct nucleus with a specific number of protons and electrons is known as a nuclide. Nuclides in nuclear reactions are usually written as follows:
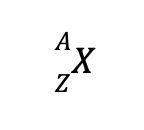
where X is the element’s identity, A is the mass number (sum of protons and neutrons), and Z is the atomic number, also known as the proton number.
The first thing you need to know before we start any discussion is how to count protons, neutrons, and electrons in an atom. Atomic number (Z) will readily give you the number of protons. In a neutral atom, the net charge is zero. Therefore, there are equal numbers of protons and electrons in a neutral atom. Lastly, you can get the number of neutrons by subtracting the proton number from the mass number. In equation form:
No. of protons = No. of electrons = Z (in a neutral atom)
No. of neutrons = Mass number (A) – Atomic number (Z)
An atomic number is a unique number for a specific element. You can see the atomic number written on top of the element symbol in the periodic table of elements.
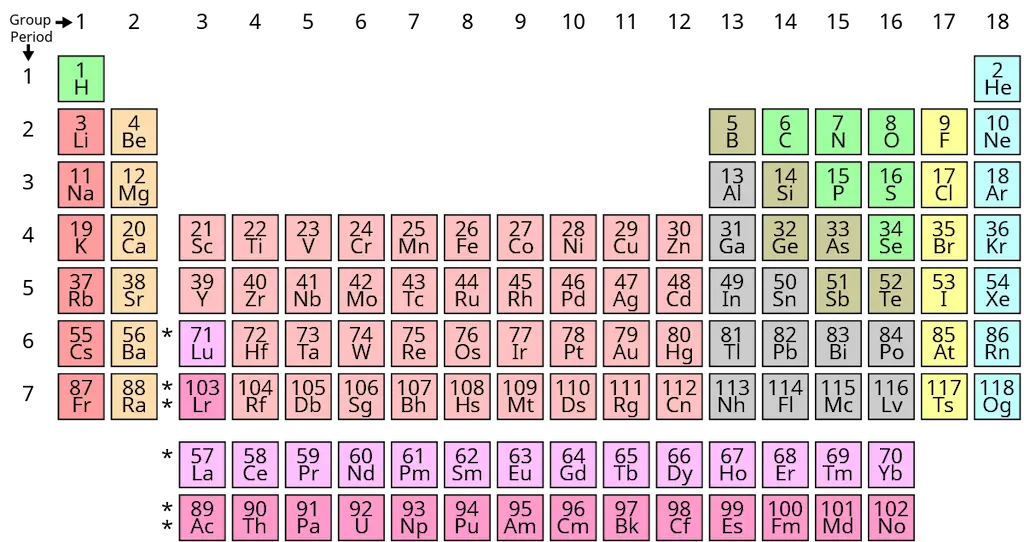
As you can see, other than carbon, no other element in the periodic table has an atomic number of 6. Changing the atomic number will then result in a change in the identity of the element.

Although the atomic number is unique, there are nuclides of the same element with the same number of protons but differ in the number of neutrons. Such nuclides are called isotopes. One example is the isotopes of hydrogen:

As you can see in the illustration below, these hydrogen atoms all have 1 proton in their nucleus and 1 electron around it but have 0, 1, and 2 neutrons, respectively.
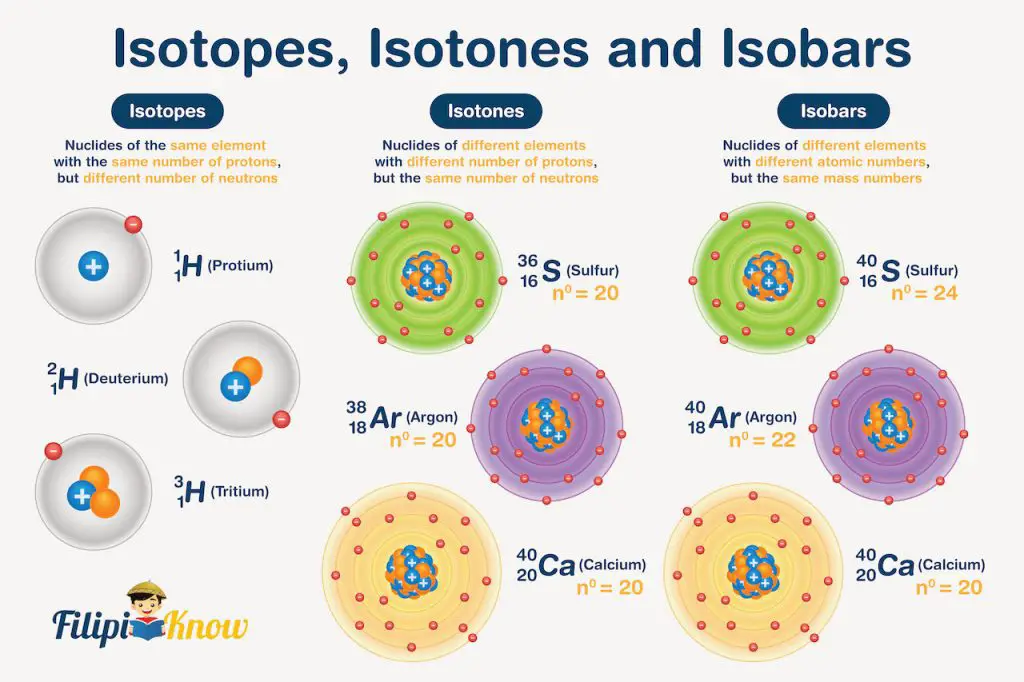
In contrast, atoms with the same number of neutrons but different numbers of protons are called isotones. Examples are the following:
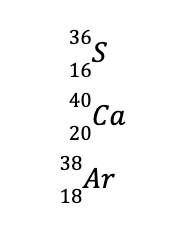
If you try to determine the nucleons in these nuclides, they all have 20 neutrons and 16, 20, and 18 protons, respectively.
Lastly, isobars are nuclides with the same mass number but differ in their atomic numbers, like the following:
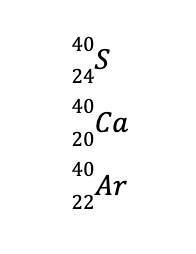
Balancing Nuclear Reactions
Balancing nuclear reactions entails familiarizing oneself with different notations used in nuclear chemistry. The table below provides the summary of all these notations.
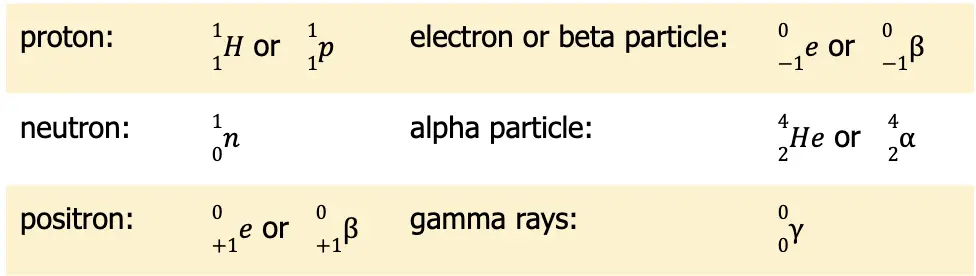
Now that you know all these notations, you should be able to balance nuclear reactions. Among all the reactions that you will encounter, this type of reaction is the easiest to balance. There are only two rules:
- The sum of the subscripts on the reactant side must equal the sum of the subscripts on the product side.
- The sum of the superscripts on the reactant side must equal the sum of the superscripts on the product side.
Sample Problems:
Balance the following nuclear reactions by providing the identity of the unknown nuclide and/or particle.
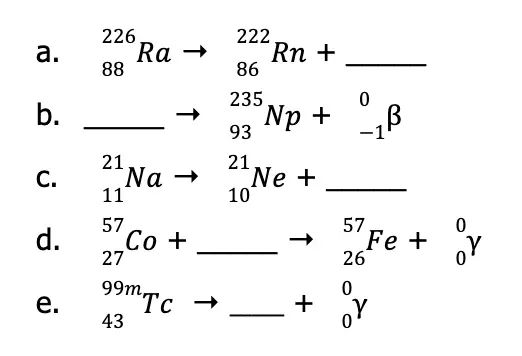
Solution:
a. To balance the reaction, the superscript on the product side should be equal to 226, while the subscript of the product side should be equal to 88.
Hence, the missing species for us to balance the equation is
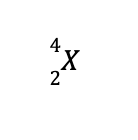
From the notations previously presented, this corresponds to an alpha particle, which can be represented by either
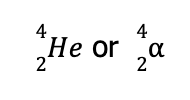
Hence, the balanced nuclear reaction is
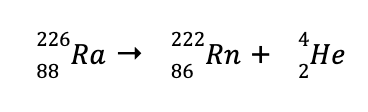
A type of nuclear reaction which leads to the emission of alpha particles is called alpha particle emission or alpha decay.
Additional Info: In a nuclear reaction, the atom on the reactant side is called the parent nuclide, while the atom/s on the product side is called the daughter nuclide/s.
b. Using the same technique in (a), we can determine the missing species to balance the reaction is
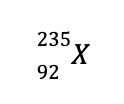
Looking at the periodic table, the element with an atomic number of 92 is U (remember that the atomic number is unique for every element). Hence, the balanced nuclear reaction is
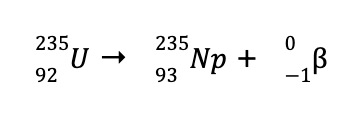
Such a reaction wherein a beta particle was produced is called a beta particle emission or beta decay.
c. The missing species to balance the reaction is
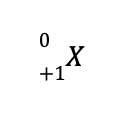
This means that the identity of the missing species is a positron, which is designated by
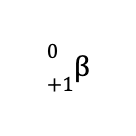
As a result, the balanced nuclear reaction is
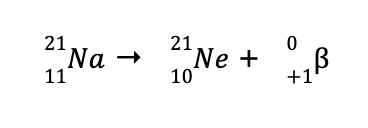
When a positron is emitted during a nuclear reaction, the reaction is called positron emission or positron decay.
d. The missing species to balance the reaction is
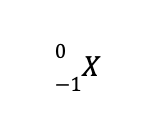
This means that the identity of the missing species is an electron, which is denoted by
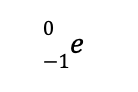
As a result, the balanced nuclear reaction is
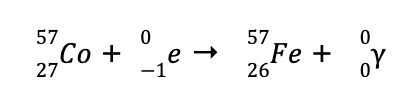
When a gamma emission is accompanied by the capture of electrons, the reaction is called an electron capture reaction.
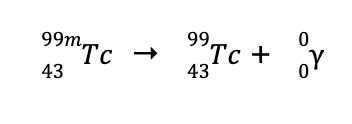
e. The “m” in the mass number indicates that the nuclide is in a metastable state. Metastable nuclides are very unstable. To increase its stability, it undergoes gamma decay, which results in the formation of the daughter nuclide with the same mass and atomic number as the parent nuclide, but is more stable. Hence, the balanced nuclear reaction is
Calculation of Half-Life
By definition, half-life (designated as t½ or t0.5) is the length of time needed for the amount of a radioactive nuclide to decrease by one-half of its previous amount. All radioactive reactions follow the first order rate law expression, and for all 1st order reactions, the half-life can be calculated as follows:
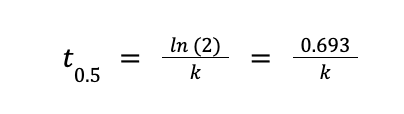
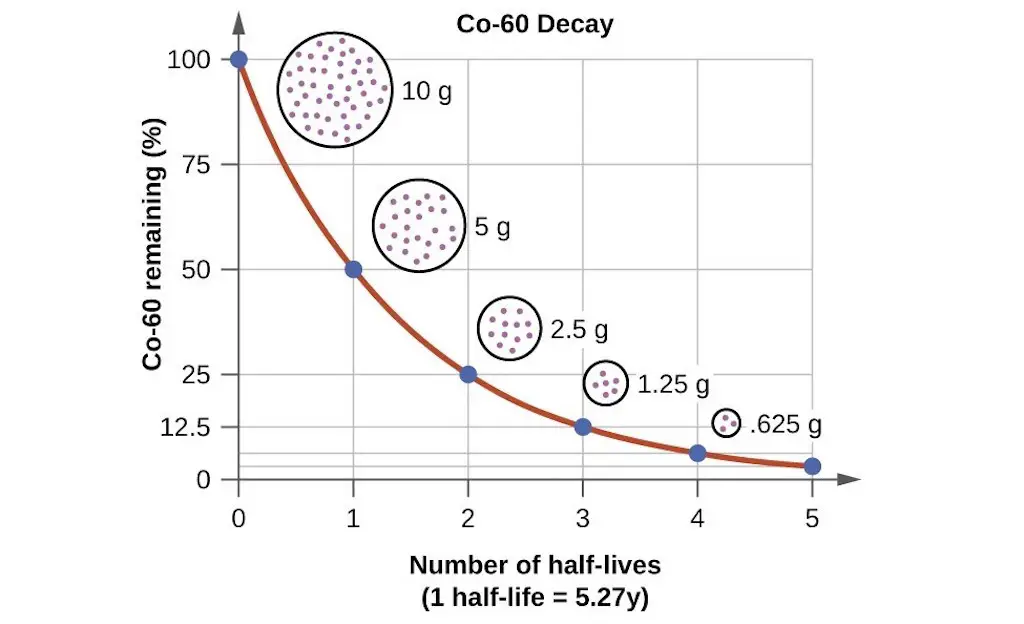
where k is the rate constant of the reaction. The figure below demonstrates the concept of half-life.

Sample Problem: Derive an expression that will allow us to solve the rate of decay of 131I if its half-life is 8 days.
Solution:
From the equation to solve for half-life:
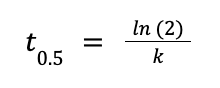
Solving for k, we have
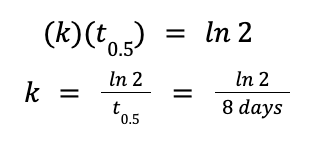
References
Chang, R. (2010). Chemistry (10th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Silberberg, M. (2006). Chemistry: The molecular nature of matter and change (4th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Zumdahl, S., Zumdahl, S., & DeCoste, D. (2010). Chemistry (8th ed.). Belmont, California: Brooks Cole.
Next topic: Concentration of Solutions
Previous topic: Thermochemistry
Return to the main article: The Ultimate Chemistry Reviewer
Download Article in PDF Format
Test Yourself!
1. Practice Questions [PDF Download]
2. Answer Key [PDF Download]
Written by John Bryan Rolloque
in College Entrance Exam, LET, NMAT, Reviewers, UPCAT
John Bryan Rolloque
John Bryan Rolloque graduated cum laude at the University of the Philippines Los Baños in 2018 under the B.S. Agricultural Chemistry program. He taught courses in general chemistry, analytical chemistry, and organic chemistry at UPLB’s Institute of Chemistry, and has been serving as the Region IV coordinator for the Regional and National Chemistry Olympiad. Landing 8th place in the 2019 licensure exam for agriculturists, he is now taking up his master’s degree in plant physiology, also in UPLB.
Copyright Notice
All materials contained on this site are protected by the Republic of the Philippines copyright law and may not be reproduced, distributed, transmitted, displayed, published, or broadcast without the prior written permission of filipiknow.net or in the case of third party materials, the owner of that content. You may not alter or remove any trademark, copyright, or other notice from copies of the content. Be warned that we have already reported and helped terminate several websites and YouTube channels for blatantly stealing our content. If you wish to use filipiknow.net content for commercial purposes, such as for content syndication, etc., please contact us at legal(at)filipiknow(dot)net