Reversible Reactions and Chemical Equilibria
Most of the reactions that we’ve presented so far are those that essentially proceed to completion. As a result, they are represented using a unidirectional arrow that points to the products. However, did you know that the majority of reactions are reversible, at least to some extent? In this topic, you will learn more about reversible reactions (aka equilibrium reactions).
Click below to go to the main reviewers:
Table of Contents
- The Le Chatelier’s Principle
- Factors Affecting Equilibrium
- Writing Equilibrium Constant Expressions
- References
- Download Article in PDF Format
- Test Yourself!
The Le Chatelier’s Principle
When reactions proceed to completion, it is represented in a chemical reaction using the unidirectional arrow. Such reactions are called irreversible reactions, and the reactants will be converted to products until the depletion of the limiting reagent.
On the other hand, some reactions are reversible. Reversible (or equilibrium) reactions are depicted using bidirectional harpoons (⇌), denoting that both forward and reverse reactions occur simultaneously. When the rate of the forward reaction equals the rate of the reverse reaction and the concentrations of the reactants and products remain constant, then the state of chemical equilibrium is achieved.
There are certain events that disturb the dynamic equilibrium of a chemical system. The effect(s) of such disturbances can be predicted by Le Chatelier’s principle, which states that if an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches the new equilibrium position.
Factors Affecting Equilibrium
Changes in concentration, temperature, volume, pressure, and the presence of a catalyst affect a chemical system at equilibrium. To demonstrate the effect of each factor, let us look at several examples.
First, to investigate the effect of concentration, consider the dissociation of acetic acid (CH3COOH) in water as shown below:
CH3COOH(aq) + H2O(l) ⇌ CH3COO—(aq) + H3O+(aq)
As you can see, a bidirectional harpoon was used to represent the reaction. It means it’s reversible, and the forward and backward reactions occur simultaneously.
Now, imagine that you’ve added a few drops of CH3COOH to the container. By doing so, you are introducing stress into the system. You might ask: WHY? Well, the main reason is that at equilibrium, the concentration of all the species involved (reactants and products) is constant. By adding a few drops of CH3COOH, you are basically changing the concentration of CH3COOH in the system, which causes the system to become stressed.
Just like us, systems at equilibrium have coping mechanisms to lessen/remove the stress introduced to them. Reversible reactions deal with stresses by favoring either the forward or the reverse reaction until the system reaches a new equilibrium position. In this specific example, the concentration of CH3COOH increases, so to achieve equilibrium again, the system will reduce the concentration of CH3COOH by momentarily favoring the forward reaction. During this adjustment, the rate of the forward reaction is greater than the rate of the reverse reaction. However, once the new equilibrium position is reached, the rate of the forward and reverse reactions will once again be equal.
The same is true if, for example, you added a pint of NaCH3COO powder to increase the concentration of CH3COO—. This time, since CH3COO— is on the right side of the equilibrium reaction, the reverse reaction will be momentarily favored to reduce the total concentration of CH3COO— until such time that a new equilibrium position is reached.
In our next scenario, what if for some reason, we decided to remove H3O+? How will the system react? Well, since one of the species on the right side of the reaction is being removed, the system needs to replenish it to somehow counteract the stress. To do so, the reaction will momentarily favor the forward reaction (the reaction shifts to the right) until a new equilibrium position is reached to compensate for the H3O+ that was removed.
Lastly, what would happen if we added a few drops of water to the container? Based on our discussion so far, you might have guessed that the reaction will shift to the right to reduce the amount of water added. However, that is not the case. Only aqueous and gaseous species can affect a system at equilibrium. Solids and liquids, regardless of amount, do not cause a change in equilibrium position. In our example, water is in liquid form, therefore, changing its concentration will not cause any change in the equilibrium.
Let’s go to the next factor which is pressure. Changes in pressure are closely related to changes in the volume of the system. To help you understand the discussion, refer to the image shown below.
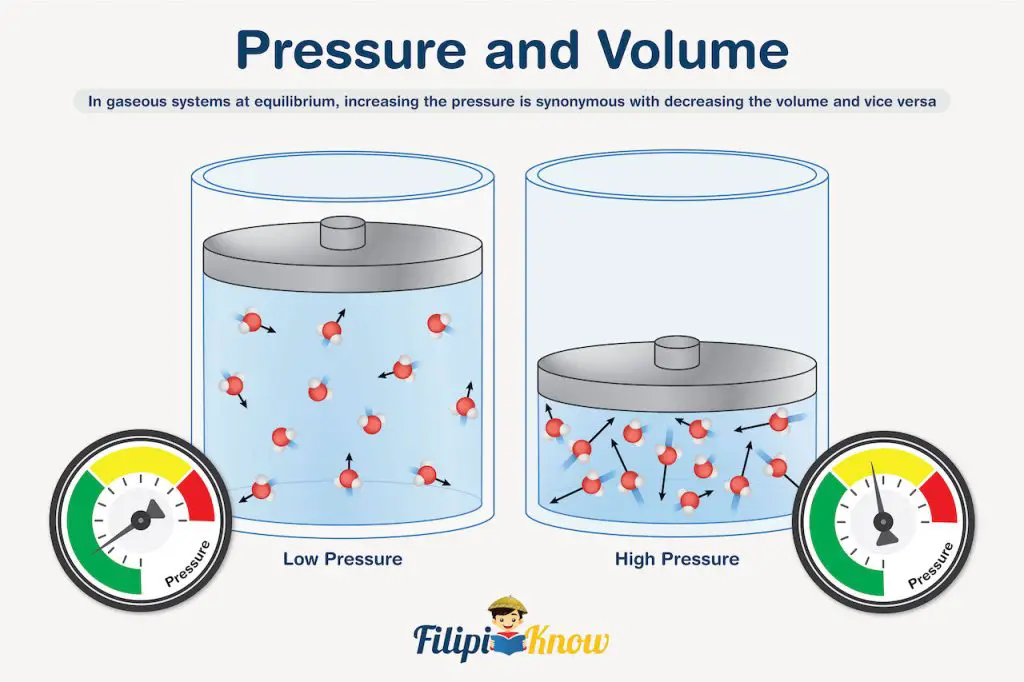
You can see that both scenarios consider the same amount of gas, which, for this example, is exactly 12 molecules. Notice that increasing the pressure applied to the gas causes a decrease in the volume occupied by the gas. Therefore, we can say that in gaseous systems at equilibrium, increasing the pressure is synonymous with decreasing the volume and vice versa. This also implies that changes in pressure (and volume) only affect gaseous systems simply because aqueous solutions, liquids, and solid reagents are incompressible.
Let us consider the very famous reaction of N2O4 in demonstrating the effect of pressure in gaseous systems at equilibrium. The reaction is written as:
N2O4(g) ⇌ 2NO2(g)
What’s so special about this reaction is that you can actually see the system reacting to changes in pressure.
How?
Well, N2O4 is a colorless gas, while NO2 is a brown gas. During expansion (reduced pressure and higher volume), the reaction favors the formation of gas with a higher number of moles since there is a larger space that the gas can occupy. In our example, there are 2 moles of NO2 on the right side of the reaction and 1 mole of N2O4 on the left. Since NO2 has a greater number of moles than N2O4, the equilibrium shifts to the right during expansion, and visually, what you will see inside the syringe is a gas that is initially nearly colorless (since it is first dominated by colorless N2O4) that turns darker brown due to the equilibrium shift favoring the formation of NO2.
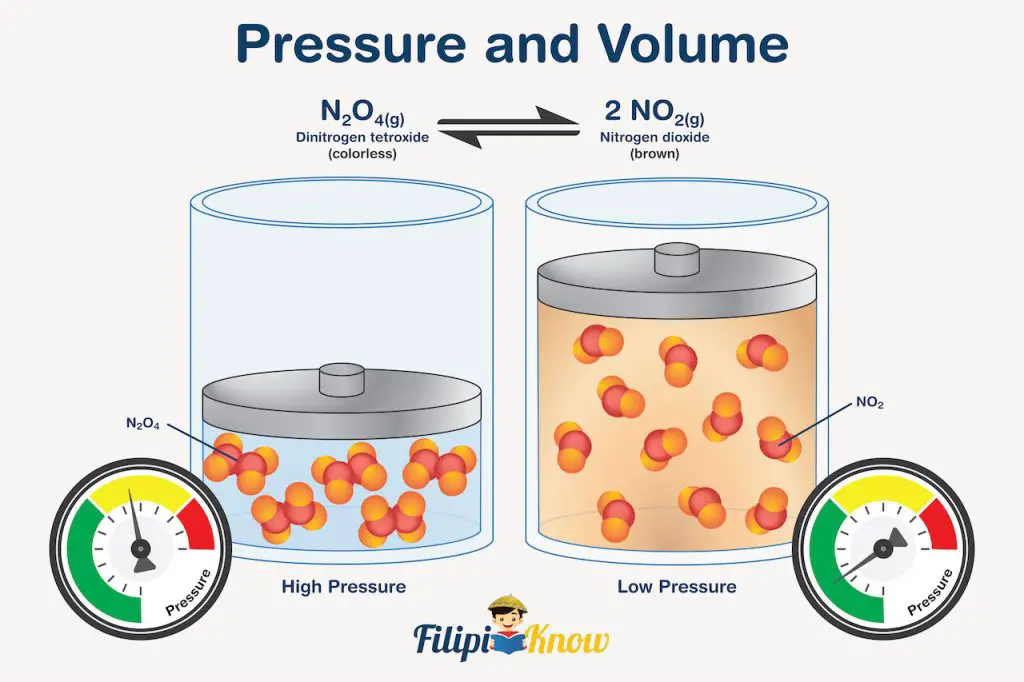
The opposite is true during compression. The increase in pressure reduces the volume the gas occupies, and the equilibrium reaction shifts toward the formation of gas with fewer moles. In our particular example, equilibrium will shift towards the formation of colorless N2O4, and you will see it visually in the syringe as a change in color from brown to nearly colorless after compression. This change in color signifies that more N2O4 exists compared to NO2 after compression.
Another parameter that affects equilibrium reaction is temperature. Changes in temperature, however, only affect heat-releasing (exothermic) and heat-absorbing (endothermic) reactions. For such cases, the heat released is treated as if it is a product of the chemical reaction, while the heat absorbed is treated as if it is a reactant.
Since we are talking about equilibrium reactions, if the forward reaction is endothermic (ΔH° > 0), then the reverse is exothermic (ΔH° < 0). Take note, however, that there is no such thing as negative heat. When you encounter a negative ΔH°, the negative sign only implies that the heat was released. On the other hand, if you encounter positive ΔH°, it means that heat was absorbed by the system.
Let us consider again the reaction of N2O4 and NO2 but this time, let us incorporate heat. The complete reaction is as follows:
heat + N2O4(g) ⇌ 2NO2(g)
As you can see, heat is written on the reactant side, so the forward reaction is actually heat-absorbing or endothermic. If heat is written on the product side, the reaction is exothermic. Therefore, the reverse reaction is an exothermic process.
To determine the effect of temperature on equilibrium reactions, we need to treat heat as if it is a reagent. For example, if we increase the temperature, it can be seen as if we added heat to the system. To compensate for this stress, the reaction will shift to the right, favoring the formation of brown NO2 gas. If you do the reverse, it can be viewed as if we remove heat from the system; hence, the reaction will shift to the left, favoring the formation of colorless N2O4 gas. The illustration shown below will help you understand this better.
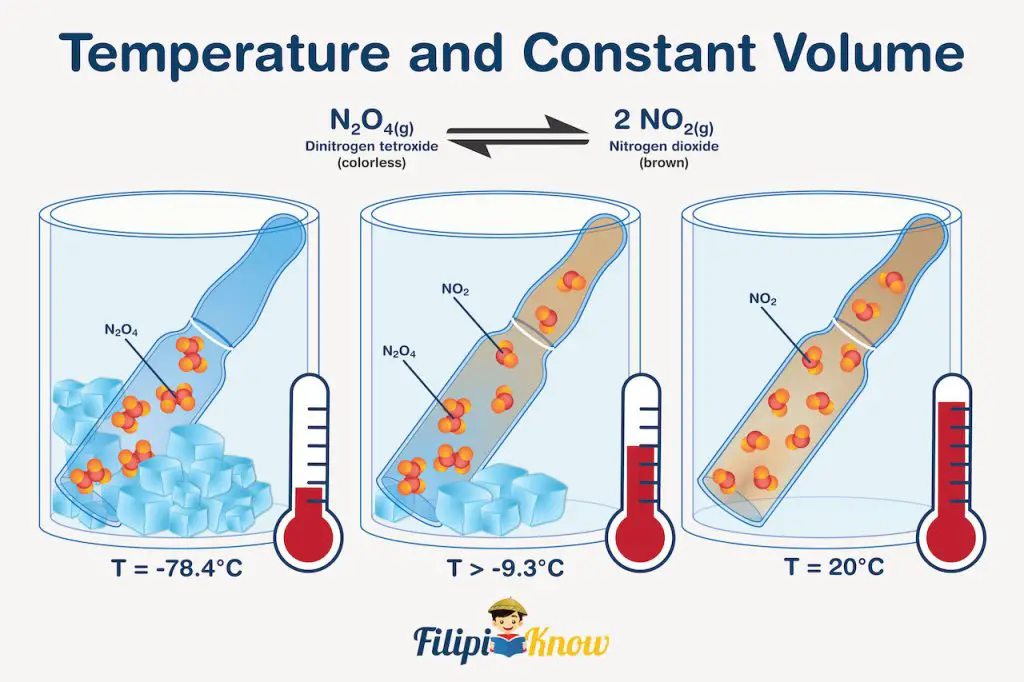
In this figure, N2O4 and NO2 were also used, but instead of a syringe, the system was contained inside a sealed glass bulb. In (a) (T = -78.4 °C), the low temperature implies that it’s as if we are removing heat from the system, thereby shifting the equilibrium towards the formation of colorless N2O4 gas which solidifies due to extremely low temperature. True enough, no colored gas is visible inside the bulb.
Meanwhile, in (c) (T = 20 °C), it’s as if the system was allowed to absorb heat, and the equilibrium shifts toward the formation of brown NO2 gas, which is very evident due to the reddish-brown color of the gas inside the bulb. In (b) (T = -9.3 °C), the bulb contains light yellow gas, which only indicates that appreciable concentrations of both N2O4 and NO2 exist inside the bulb.
Lastly, we will study the effect of catalysts on equilibrium reactions. There is a common misconception that catalysts increase the speed of chemical reactions by lowering the activation energy. In reality, a catalyst increases the rate of a chemical reaction by providing an alternative reaction pathway with a lower energy of activation.
When applied in equilibrium reactions, the catalyst increases the rate of the forward reaction. However, it also increases the rate of the reverse reaction. Hence, the catalyst does not alter the equilibrium constant nor shift the equilibrium reaction. Now, let us work with an example.
Sample Problem:
Determine the effect of the following conditions on the hypothetical equilibrium reaction shown below. Briefly explain the effect that you selected.
A(s) + B(aq) + 2C(g) + heat ⇌ D(g) + E(l)
Solution:
| CONDITION | EFFECT | EXPLANATION |
| ↑P | shifts to the right | High pressure entails lower volume; shifts toward the side with a lower number of gas molecules formed |
| ↓[B] | shifts to the left | Reactants were removed; shifting the equilibrium to the left to compensate for the removal of B |
| ↓T | shifts to the left | Heat is being removed; shift towards the side where heat is being formed to compensate for the heat that is being removed |
| ↑[E] | no effect | Changes in the concentration of liquid species do not affect the equilibrium |
| ↑V | shifts to the left | Increasing the volume result in a shift towards the side with a larger number of gas molecules formed |
| ↑[A] | no effect | Changes in the concentration of solid species do not affect the equilibrium |
| catalyst was added | no effect | Catalysts have no net effect on equilibrium reactions |
Writing Equilibrium Constant Expressions
Consider the hypothetical reaction
aA(aq) + bB(g) ⇌ cC(g) + dD(aq)
where A and B are the reactants, C, and D are the products, and a, b, c, and d are the numerical coefficients in a balanced chemical reaction. For any equilibrium reaction, an expression for the equilibrium constant expression can be written as follows:
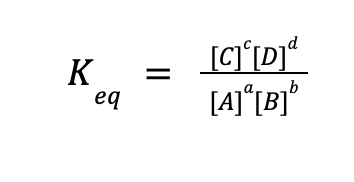
In simple terms, the Keq expression is equal to the product of the equilibrium concentration (or partial pressure for gaseous species) of the products raised to their coefficients in a balanced chemical reaction, all over the product of the equilibrium concentration (or partial pressure for gaseous species) of the reactants raised to their coefficients in a balanced chemical reaction.
Take note that only aqueous and gaseous species must be included in the equilibrium constant expression. Solids and liquids are not included because their concentrations are so large that it is almost unchanged in any equilibrium reactions.
Sample Problem:
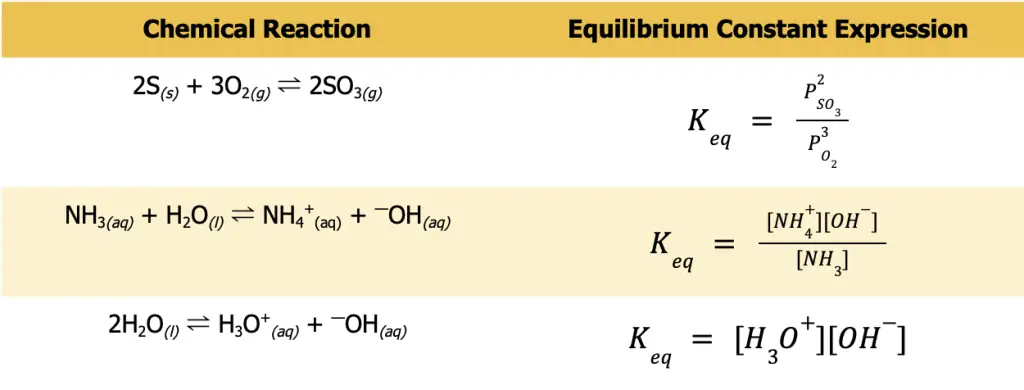
Write the equilibrium constant expression for the following sets of reactions.
- S(s) + O2(g) ⇌ SO3(g)
- NH3(aq) + H2O(l) ⇌ NH4+(aq) + —OH(aq)
- H2O(l) ⇌ H3O+(aq) + —OH(aq)
Solution:
The first thing you need to do before writing any Keq expression is to ensure that the reaction you are considering is balanced. Otherwise, you will not be able to write the expression correctly. Also, always remember that only the gaseous and aqueous species must be included in the Keq expression. Furthermore, take note that for gaseous species, P is more appropriate to use to denote that you are pertaining to the partial pressure of the gas.
Meanwhile, square brackets are more appropriate for aqueous solutions to signify that it is the concentration that is being considered. The table below gives the balanced chemical reaction and the appropriate Keq expression.
References
Chang, R. (2010). Chemistry (10th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Silberberg, M. (2006). Chemistry: The molecular nature of matter and change (4th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Zumdahl, S., Zumdahl, S., & DeCoste, D. (2010). Chemistry (8th ed.). Belmont, California: Brooks Cole.
Next topic: Acids and Bases
Previous topic: Chemical Kinetics
Return to the main article: The Ultimate Chemistry Reviewer
Download Article in PDF Format
Test Yourself!
1. Practice Questions [PDF Download]
2. Answer Key [PDF Download]
Written by John Bryan Rolloque
in College Entrance Exam, LET, NMAT, Reviewers, UPCAT
John Bryan Rolloque
John Bryan Rolloque graduated cum laude at the University of the Philippines Los Baños in 2018 under the B.S. Agricultural Chemistry program. He taught courses in general chemistry, analytical chemistry, and organic chemistry at UPLB’s Institute of Chemistry, and has been serving as the Region IV coordinator for the Regional and National Chemistry Olympiad. Landing 8th place in the 2019 licensure exam for agriculturists, he is now taking up his master’s degree in plant physiology, also in UPLB.
Copyright Notice
All materials contained on this site are protected by the Republic of the Philippines copyright law and may not be reproduced, distributed, transmitted, displayed, published, or broadcast without the prior written permission of filipiknow.net or in the case of third party materials, the owner of that content. You may not alter or remove any trademark, copyright, or other notice from copies of the content. Be warned that we have already reported and helped terminate several websites and YouTube channels for blatantly stealing our content. If you wish to use filipiknow.net content for commercial purposes, such as for content syndication, etc., please contact us at legal(at)filipiknow(dot)net