Chemical Reactions

Did you know that sodium metal reacts very violently with water, to the point that an explosion is most likely to occur when they come in contact with one another? Meanwhile, chlorine gas can cause poisoning which leads to nausea, vomiting, and pulmonary problems like violent cough and asthma. Combine them, and voila! You will have NaCl, more commonly known as table salt.
Can you believe that two very dangerous elements, when combined, can add flavor to the food you eat? Well, it’s all thanks to what we know as chemical reactions. Learn more about chemical reactions in this module.
Click below to go to the main reviewers:
Table of Contents
- Definition of Chemical Reactions
- Types of Chemical Reactions
- Balancing Chemical Reactions
- Reaction Stoichiometry: Limiting and Excess Reactants
- References
- Download Article in PDF Format
- Test Yourself!
Definition of Chemical Reactions
Chemical reactions are processes in which a substance (or substances) is changed into one or more substances.
In a chemical reaction, everything that is written on the left side of the arrow is called reactants, while everything that is written on the right side of the arrow is called products.
All chemical reactions obey the law of conservation of matter proposed by Antoine Lavoisier in the 18th century. It states that “matter can neither be created nor destroyed.” This implies that mass is conserved in any chemical reaction. For example, if a 10 g reactant/s undergoes a reaction that proceeds to completion, then the product/s formed should weigh exactly 10 g.
Types of Chemical Reactions
1. Combination Reaction
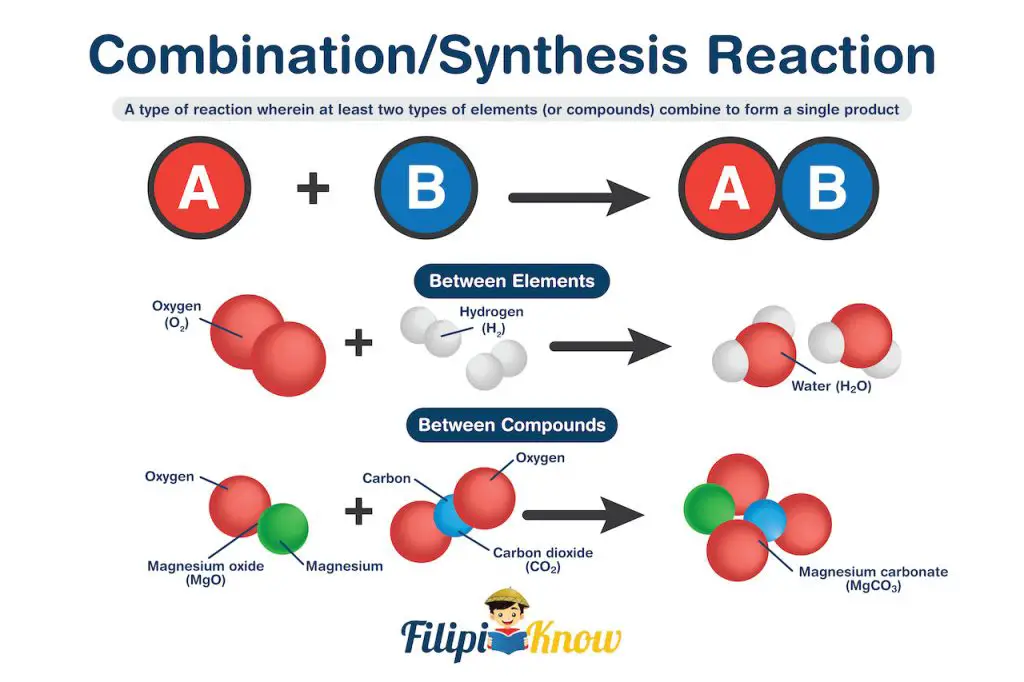
Also known as the synthesis reaction, the combination reaction is a type of reaction wherein at least two types of elements (or compounds) combine to form a single product.
2. Decomposition Reaction
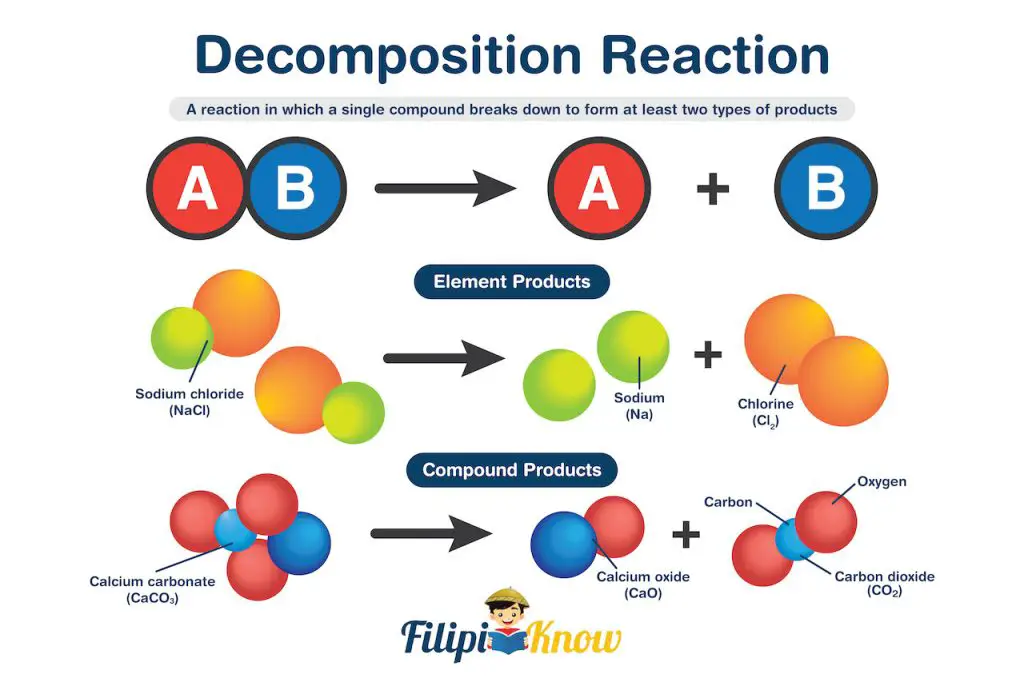
A decomposition reaction is a type of reaction in which a single compound breaks down to form at least two types of products.
3. Single Displacement Reaction
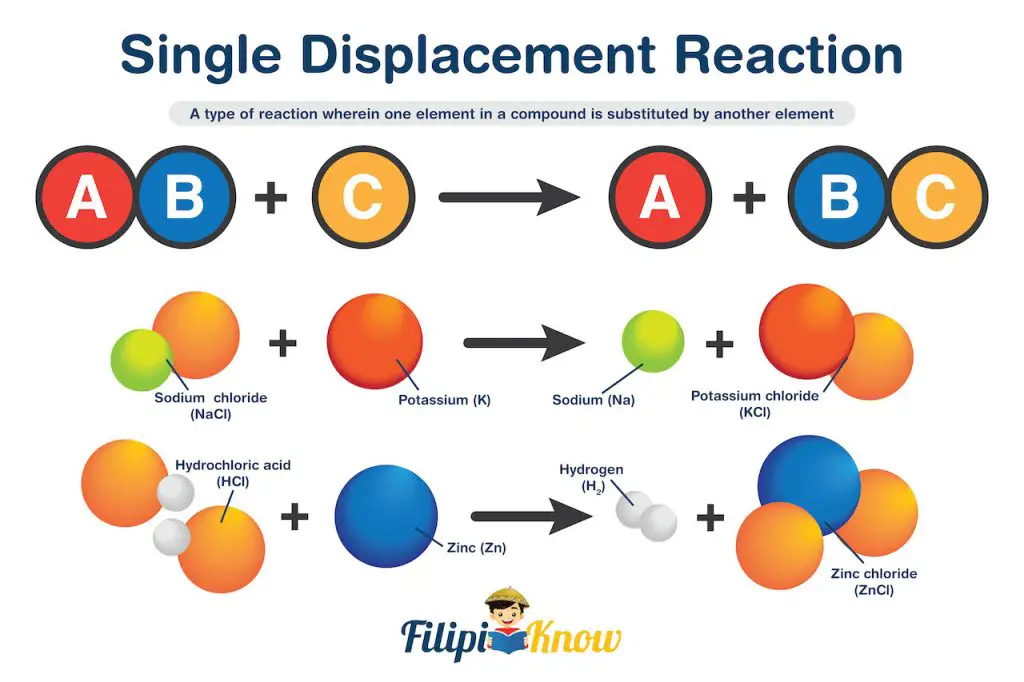
Also known as the single replacement reaction, this is a type of reaction wherein one element in a compound is substituted by another element.
4. Double Displacement Reaction
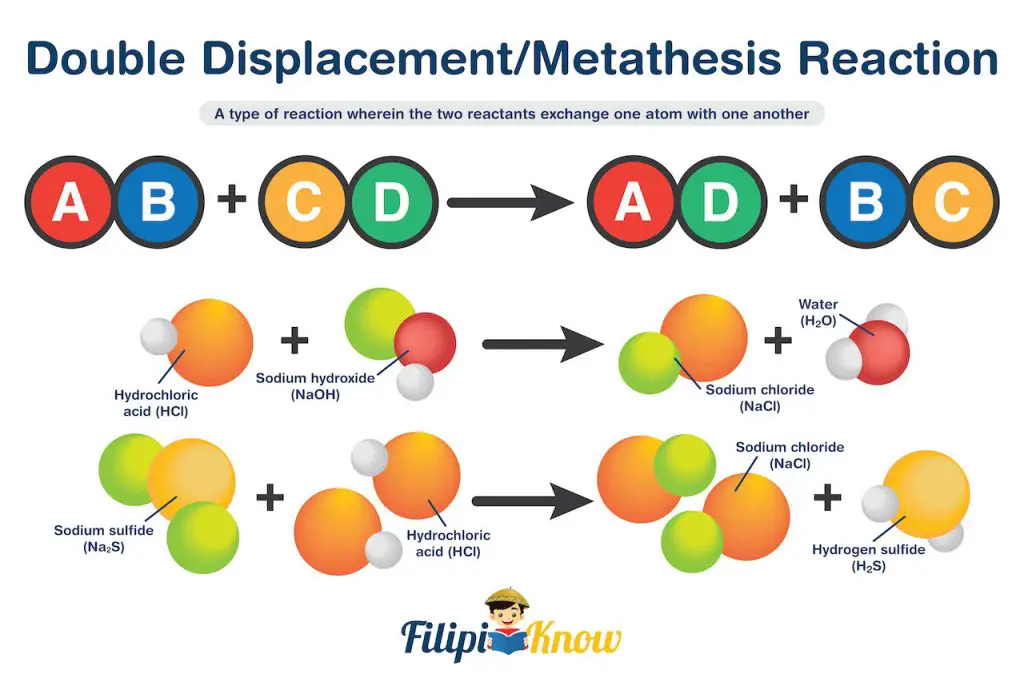
Also known as metathesis reaction, this is a type of reaction wherein the two reactants exchange one atom.
5. Redox Reaction
This is a type of reaction that involves the transfer of electrons between two species.
Redox reaction, a shorthand term for reduction-oxidation reaction, always occurs in pairs as there should be a species that will receive the electron/s lost by the other to maintain electroneutrality. An example of a redox reaction is shown below.
2Al(s) + 3Cu2+(aq) ⟶ 2Al3+(aq) + 3Cu(s)
You can see that the oxidation state of Al changes from 0 to +3 (rules in assigning oxidation states were discussed in a previous article), which means that Al loses electrons (oxidation). On the other hand, the oxidation state of Cu changes from +2 to 0, which means that Cu gains electrons (reduction). It is confusing to recognize which species undergoes reduction and oxidation, but there are two simple mnemonics that can help you:
GEROA (Gain of Electron/s, Reduction, Oxidizing Agent)
LEORA (Loss of Electron/s, Oxidation, Reducing Agent)
As we know, electrons are negatively charged. Therefore, you will recognize the gain of electrons if the oxidation number decreases. In contrast, you will recognize the loss of electrons if the oxidation number increases.
ROD (Reduction, Oxidation number Decreases)
In some instances, only one species undergoes both oxidation and reduction. Such a reaction is a special redox reaction more commonly known as a disproportionation reaction. An example is shown below. Note that the oxygen atom undergoes both reduction (from -1 to -2) and oxidation (from -1 to 0).
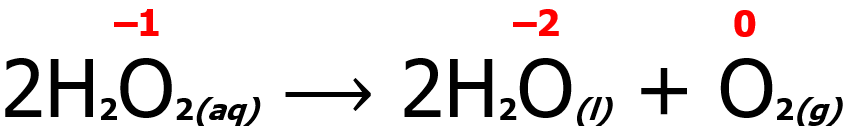
Meanwhile, some redox reactions have reactants containing the same element with different oxidation states but forming a product wherein the same element has the same oxidation number. Such a reaction is known as a comproportionation (or synproportionation) reaction. An example is shown below. Note that two Ag atoms with different oxidation states react with each other to form a single Ag atom with an oxidation state of +1. Furthermore, Ag undergoes both reduction (from +2 to +1) and oxidation (from 0 to +1).
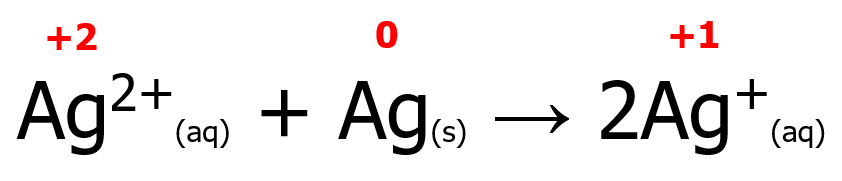
6. Combustion Reaction
A combustion reaction is a reaction in which flammable compounds (usually organic compounds) react with oxygen from the atmosphere.
If the atmosphere is oxygen-rich, complete combustion occurs, and the sole products are CO2(g) and H2O(g) for organic compounds containing only C and H (also known as hydrocarbons). If the atmosphere is oxygen-deficient, incomplete combustion occurs, and CO(g) and C(s) also form in addition to CO2(g) and H2O(g).
Furthermore, nitrogen-containing organic compounds form oxides of nitrogen, mainly NO2(g) upon combustion; sulfur-containing organic compounds form SO2(g); while halogenated organic compounds form gaseous HX, where X can be F, Cl, Br, or I. Some examples are shown below.
C6H14 + 19⁄2O2(g) ⟶ 6CO2(g) + 7H2O(g) + heat
C6H13Cl + 9O2(g) ⟶ 6CO2(g) + 6H2O(g) + HCl(g) + heat

7. Precipitation Reaction
This is a reaction in which the mixing of two solutions results in the formation of solids, known as a precipitate. An example is shown below:
Pb(NO3)2(aq) + 2NaCl(aq) ⇌ PbCl2(s) + 2NaNO3(aq)
8. Neutralization Reaction
Also known as an acid-base reaction, a neutralization reaction is a reaction between an acid and a base. Such reactions always result in the formation of water and salt. An example is shown below:
HCl(aq) + NaOH(aq) ⟶ NaCl(aq) + H2O(l)
You should have noticed that precipitation and neutralization reactions are special types of double displacement reactions.
Balancing Chemical Reactions
Balancing a chemical reaction is one of the fundamental tasks that you need to do in chemistry. Some reactions are relatively easy to balance, while others are more challenging. Let us work with some examples to demonstrate how to balance chemical reactions.
Sample Problem 1
Balance the following chemical reaction.
KClO3 ⟶ KCl + O2
Solution
All chemical reactions follow the law of conservation of matter which states that matter can neither be created nor destroyed. Hence, the number of atoms of a certain element in the reactant and product side must be the same. With this, it is only logical that the first step in balancing any chemical reaction is to count the total number of distinct atoms on the reactant side and the product side.
For beginners, it is a good idea to construct a table like what is shown below.
| Atom | Reactant Side | Product Side |
| K | 1 | 1 |
| Cl | 1 | 1 |
| O | 3 | 2 |
You can immediately see that the reaction is not balanced since there are only 2 oxygen atoms on the product side, while there are 3 on the reactant side.
Most references will recommend trial and error until you find the right combination of coefficients. However, during an examination where time is of the essence, doing that can be counterproductive and time-consuming, especially if the reaction is complicated. A faster way to balance reactions like this is to use fractions at first to balance the atoms that are not equal on the reactant and product side. In this example, I can use 3⁄2 as the coefficient of O2 on the product side as follows:
KClO3 ⟶ KCl + 3⁄2O2
Reconstructing the table above, we’ll see that the reaction is balanced already.
| Atom | Reactant Side | Product Side |
| K | 1 | 1 |
| Cl | 1 | 1 |
| O | 3 | (3⁄2)(2) = 3 |
However, you will rarely see fractions as numerical coefficients of chemical reactions simply because there’s no such thing as 1.5 oxygen gas (just like there’s no such thing as ½ human). To eliminate fractions, what you can do is multiply all the coefficients with the least common multiple of all the denominators.
In our example, we can multiply all the numerical coefficients by 2.
2 [KClO3 ⟶ KCl + 3⁄2O2] 2
2KClO3 ⟶ 2KCl + 3O2
As you can see, we were able to balance the chemical reaction without actually going through the laborious process of trial and error!
Sample Problem 2
Shown below is the reaction for the complete combustion of butane. Write the balanced chemical reaction.
C4H10 + O2(g) ⟶ CO2(g) + H2O(g) + heat
Solution
Following the steps given in the first problem:
| Atom | Reactant Side | Product Side |
| C | 4 | 1 |
| H | 10 | 3 |
| O | 2 | 2 |
Even without constructing the table, it is very evident that the reaction is not balanced. For cases like this, you should first balance the atoms that appear only once on the reactant and product side. For example, C appears only once in the reactant (in C4H10) and product (in CO2). The same is true for H which appears only in C4H10 in the reactant and H2O in the product. Hence, we should balance these atoms first, giving us the reaction below:
C4H10 + O2(g) ⟶ 4CO2(g) + 5H2O(g) + heat
We can now balance the O atom, which appears twice on the product side (in CO2 and H2O). So far, we have 2 O atoms on the reactant and 13 O atoms on the product side. What we can do to balance the O atom is to assign 13/2 as the coefficient of O2 on the reactant side, which gives us the balanced reaction shown below. Take note that heat is a form of energy, so it can never be balanced in the manner we’ve discussed.
1C4H10 + 13⁄2O2(g) ⟶ 4CO2(g) + 5H2O(g) + heat
If in case the coefficient above (printed in red) is nowhere to be found in the choices, what you can do is convert all the coefficients to whole numbers, which can be done by multiplying all the coefficients by 2. Doing so will give the following balanced chemical reaction:
2C4H10 + 13O2(g) ⟶ 8CO2(g) + 10H2O(g) + heat
Again, as much as possible, NEVER waste your time doing trial and error in balancing chemical reactions. Instead, use fractions first, then just do the multiplication later to convert fractions to whole numbers, like what we’ve done.
Unfortunately, some reactions are hard to balance using the traditional method of counting the atoms. This is especially true for redox reactions. Furthermore, there are cases wherein you will consider the pH of the reaction matrix because the balanced chemical reaction of a certain redox reaction may vary depending on the medium’s pH.
As a result, special methods are usually used to balance redox reactions, namely the half-reaction method (also known as the ion-electron method) and the change in oxidation number method.
Sample Problem 3
Balance the redox reaction shown below under acidic and basic conditions.
MnO4—(aq) + C2O42—(aq) ⟶ Mn2+(aq) + CO2(g)
Solution
What makes balancing redox reactions slightly challenging is aside from the number of atoms, you also need to ensure that the charges and the number of electrons lost and gained are balanced.
In using the half-reaction method, the following steps must be followed:
Step 1: Assign oxidation numbers and separate the oxidation half-reaction (OHR) from the reduction half-reaction (RHR). Write the species that undergoes oxidation as the OHR, while the species that undergoes reduction must be written as RHR (recall our mnemonics earlier).
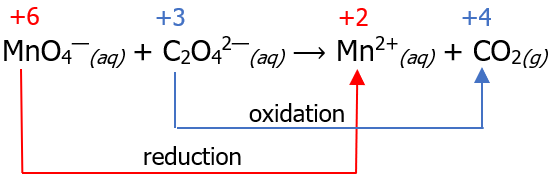
| OHR: C2O42—(aq) ⟶ CO2(g) | RHR: MnO4—(aq) ⟶ Mn2+(aq) |
Step 2: Balance all the atoms other than O and H.
| OHR: C2O42—(aq) ⟶ 2CO2(g) | RHR: MnO4—(aq) ⟶ Mn2+(aq) |
Step 3: Balance oxygen first by adding H2O.
| OHR: C2O42—(aq) ⟶ 2CO2(g) | RHR: MnO4—(aq) ⟶ Mn2+(aq) + 4H2O(l) |
Step 4: Balance hydrogen by adding H+.
| OHR: C2O42—(aq) ⟶ 2CO2(g) | RHR: MnO4—(aq) + 8H+(aq)⟶ Mn2+(aq) + 4H2O(l) |
Step 5: Balance the charge by adding e—.
| OHR: C2O42—(aq) ⟶ 2CO2(g) + 2e— | RHR: MnO4—(aq) + 8H+(aq) + 5e— ⟶ Mn2+(aq) + 4H2O(l) |
Step 6: Combine OHR and RHR by multiplying both half-reactions by a certain factor that will allow the cancellation of electrons on both reactions.
| OHR: | 5 [C2O42—(aq) ⟶ 2CO2(g) + 2e—] 5 |
| RHR: | 2 [MnO4—(aq) + 8H+(aq) + 5e— ⟶ Mn2+(aq) + 4H2O(l)] 2 |
| Overall rxn: | 5C2O42—(aq) + 2MnO4—(aq) + 16H+(aq) ⟶ 2Mn2+(aq) + 10CO2(g) + 8H2O(l) |
At this point, you already obtained the balanced reaction under acidic conditions. To obtain the balanced redox reaction under basic conditions, simply multiply both sides of the overall reaction by ―OH equal to the number of moles of H+. That will give:
16―OH [5C2O42—(aq) + 2MnO4—(aq) + 16H+(aq) ⟶ 2Mn2+(aq) + 10CO2(g) + 8H2O(l)] 16―OH
5C2O42—(aq) + 2MnO4—(aq) + 16H2O(aq) ⟶ 2Mn2+(aq) + 10CO2(g) + 8H2O(l) + 16―OH(aq)
Since there are 16 H2O on the reactant side and 8 H2O on the product side, the reaction can be further simplified as follows:
5C2O42—(aq) + 2MnO4—(aq) + 8H2O(aq) ⟶ 2Mn2+(aq) + 10CO2(g) + 16―OH(aq)
And there you have the balanced redox reaction under basic conditions. You can check if the reaction is a balanced redox reaction by counting if the number of atoms of all the elements on the reactant side is equal to the number of atoms on the product side. Another method that you can use is the change in oxidation number method.
Sample Problem 4
Balance the following redox reaction using the change in oxidation number method.
Fe2O3(s) + CO(g) ⟶ Fe(s) + CO2(g)
Solution
The first step in balancing redox reactions using this method is assigning oxidation numbers and identifying which species undergo oxidation and which undergo reduction. Then, we can connect these species by drawing an arrow.
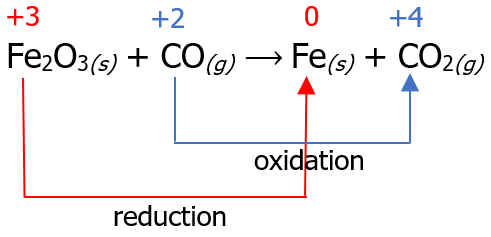
Now that we have identified the reaction that undergoes reduction and oxidation, the next step is to count the number of electrons lost in the oxidation reaction and the number of electrons gained in the reduction reaction. Then, we multiply it by a certain factor so that the number of electrons lost will be equal to the number of electrons gained.
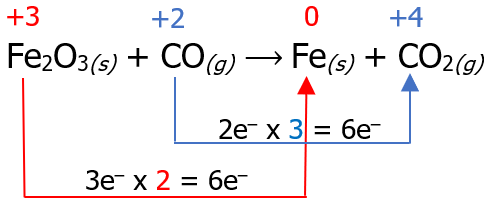
Then, place the multiplier as a coefficient of the species that undergoes oxidation and reduction reaction, but inspect if the number of atoms is the same on the reactant and product side. There are cases wherein affixing the multiplier as a numerical coefficient is not necessary, like the example below.
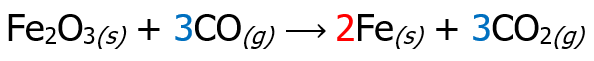
As you can see, there is no need to put 2 in front of Fe2O3 since there are 2 Fe atoms on the reactant side already. The last step is to check if the number of atoms on the reactant side is equal to the number of atoms on the product side.
Also, please bear in mind that although the change in oxidation method is relatively faster than the ion-electron method, you might find it difficult to use in some reactions, like what was given in the previous sample problem. So make sure to use appropriate methods to balance a given redox reaction.
Reaction Stoichiometry: Limiting and Excess Reactants
When chemical reactions take place, it is not always the case that the amount of the reactants involved is equal such that at the end of the reaction, no reactants will be left out. More often, one reactant will be used up first (the limiting reactant), and the others will remain unreacted (the excess reactant/s) since one of the reactants necessary for the reaction to proceed is already depleted.
Let us work with some examples to learn how to determine limiting and excess reactants.
Sample Problem 1
Exactly 8.00 g Fe2O3 (MM = 160 g/mol) reacts with 5.40 g Al (MM = 27 g/mol) to form Al2O3 (MM = 102 g/mol) and Fe (MM = 56 g/mol), according to the reaction
Fe2O3(s) + 2Al(s) ⟶ Al2O3(s) + 2Fe(s)
From this information, identify the limiting and excess reactants and calculate the mass of Al2O3 and Fe formed.
Solution
When you are presented with a chemical reaction, always check if the reaction is balanced as written. If not, you will need to balance it before calculation. Lucky for us, the reaction is balanced as written so we can proceed with the calculation.
Always bear in mind that to answer these kinds of problems correctly, you should deal with moles, not grams. Hence, the first crucial step is to convert the given mass to moles using the substance’s molar mass.
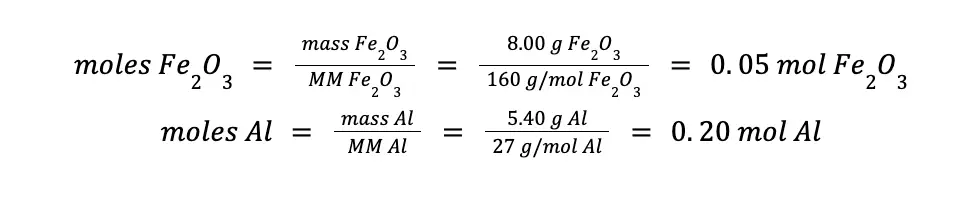
Next, considering the balanced chemical reaction, determine which reactant is excess and which one is limiting. In this example, 1 mole of Fe2O3 reacts with 2 moles of Al. Hence, a 0.05 mole Fe2O3 will require 0.10 mole Al for it to be used up completely.
Since the amount of Al present is 0.20 mole, there is more than enough Al to react with Fe2O3. Hence, the limiting reactant is Fe2O3 while the excess reactant is Al. This is despite the fact that there are 8.00 g Fe2O3 and only 5.40 g Al. So don’t let the mass fool you! Always work with moles.
To compute the mass of the products formed, we can use either the moles of Fe2O3 or moles of Al consumed. That will be:
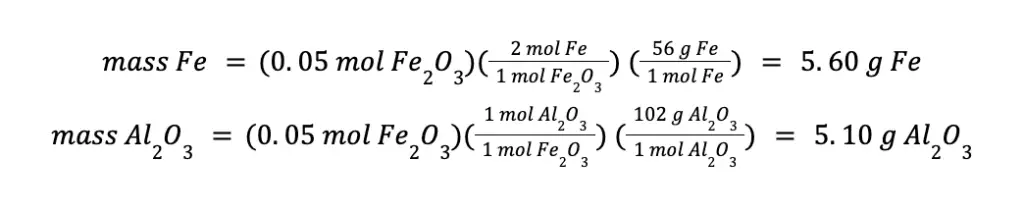
You will arrive at the same answer even if you use the mol Al consumed in your calculations.
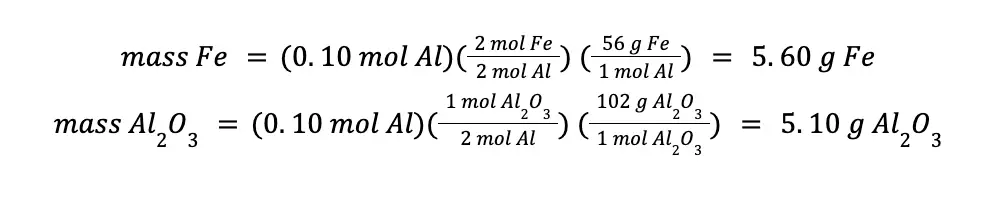
References
Chang, R. (2010). Chemistry (10th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Kotz, J. (2022). Chemistry and Chemical Reactivity. Stamford, CT: Cengage Learning.
Silberberg, M. (2006). Chemistry: The molecular nature of matter and change (4th ed.). New York City, USA: McGraw-Hill Companies, Inc.
Zumdahl, S., Zumdahl, S., & DeCoste, D. (2010). Chemistry (8th ed.). Belmont, California: Brooks Cole.
Next topic: Chemical Kinetics
Previous topic: Gases
Return to the main article: The Ultimate Chemistry Reviewer
Download Article in PDF Format
Test Yourself!
1. Practice Questions [PDF Download]
2. Answer Key [PDF Download]
Written by John Bryan Rolloque
in College Entrance Exam, LET, NMAT, Reviewers, UPCAT
John Bryan Rolloque
John Bryan Rolloque graduated cum laude at the University of the Philippines Los Baños in 2018 under the B.S. Agricultural Chemistry program. He taught courses in general chemistry, analytical chemistry, and organic chemistry at UPLB’s Institute of Chemistry, and has been serving as the Region IV coordinator for the Regional and National Chemistry Olympiad. Landing 8th place in the 2019 licensure exam for agriculturists, he is now taking up his master’s degree in plant physiology, also in UPLB.
Copyright Notice
All materials contained on this site are protected by the Republic of the Philippines copyright law and may not be reproduced, distributed, transmitted, displayed, published, or broadcast without the prior written permission of filipiknow.net or in the case of third party materials, the owner of that content. You may not alter or remove any trademark, copyright, or other notice from copies of the content. Be warned that we have already reported and helped terminate several websites and YouTube channels for blatantly stealing our content. If you wish to use filipiknow.net content for commercial purposes, such as for content syndication, etc., please contact us at legal(at)filipiknow(dot)net